Abstract
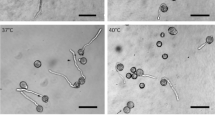
Cultured pollen protoplasts of Lilium longiflorum Thunb. were used to study, with the aid of rhodamine-phalloidin staining and immunofluorescence, changes in the organization of actin filaments and microtubules (MTs) during cell-wall regeneration, transition of cell shape from spherical to ellipsoid, growth in cell size, and pollen-tube development. In freshly isolated protoplasts, an extensive network of minute actin filaments and disorganized MTs was observed in the central cytoplasm of the vegetative cell. After regeneration of the cell wall, cortical actin filaments and MTs gradually became organized but their orientation remained random. At 5–6 d of culture, when the protoplasts changed from a spherical to an ellipsoid shape, the cortical actin filaments were aligned almost in parallel, extending between two opposite foci, and in the growing ellipsoidal cell thick actin bundles became oriented transversely to the long axis of the cell. Following the ordering of the cortical actin filaments the cortical MTs also became arranged parallel to the actin filaments. After 8–10 d of culture, when the protoplasts germinated and formed a pollen tube, both actin filaments and MTs entered the tube. Cytochalasin B and colchicine added to the medium from the beginning of the protoplast culture inhibited the organization of the actin and MT cytoskeleton, and addition of the drugs from day 5 of culture onwards destroyed the organization and arrangement of the cytoskeleton. Disruption of the actin filaments by cytochalasin B resulted also in a disruption of the MT organization and arrangement, whereas disruption of the MTs by colchicine had little effect on the organization and arrangement of the actin filaments. Both drugs blocked pollen germination in the cultured protoplasts. From these observations it is assumed that the arrangement of actin filaments plays a predominant role in pollen germination, but that the arrangement of the MTs is also essential for pollen germination through maintaining cell polarity established by the actin arrangement.
Similar content being viewed by others
Abbreviations
- MT(s):
-
microtubule(s)
References
Cass, D.D. (1973) An ultrastructural and Nomarski-interference study of the sperms of barley. Can. J. Bot. 51, 601–605
Condeelis, J.S. (1974) The identification of F actin in the pollen tube and protoplasts of Amaryllis belladonna. Exp. Cell Res. 88, 435–439
Cresti, M., Ciampolini, F., Kapil, R.N. (1984) Generative cells of some angiosperms with particular emphasis on their microtubules. J. Submicrosc. Cytol. 16, 317–326
Derksen, J., Traas, J.A. (1985) Growth of tobacco pollen tubes in vitro; effects of drug interference with the cytoskeleton. In: Proc. 8th Int. Symp. on Sexual Reproduction in Seed Plants, Ferns and Mosses, pp. 64–70, Willerase, M.T.M., Van Went, J.L., eds. Pudoc, Wageningen, The Netherlands
Derksen, J., Pierson, E.S., Traas, J.A. (1985) Microtubules in vegetative and generative cells of pollen tubes. Eur. J. Cell Biol. 38, 142–148
Franke, W.W., Herth, W., Van der Woude, W.J., Moore, D.J. (1972) Tubular and filamentous structures in pollen tubes: possible involvement as guide elements in protoplasmic streaming and vectorial migration of secretory vesicles. Planta 105, 317–341
Hasezawa, S., Hogetsu, T., Syono, K. (1989) Changes of actin filaments and cellulose fibrils in elongating cells derived from tobacco protoplasts. J. Plant Physiol. 134, 115–119
Heslop-Harrison, J., Heslop-Harrison, Y. (1989) Cytochalasin effects on structure and movement in the pollen tube of Iris. Sex. Plant Reprod. 2, 27–37
Heslop-Harrison, J., Heslop-Harrison, Y., Cresti, M., Tiezzi, A., Ciampolini, F. (1986) Actin during pollen germination. J. Cell Sci. 88, 1–8
Heslop-Harrison, J., Heslop-Harrison, Y., Cresti, M., Tiezzi, A., Moscatelli, A. (1988) Cytoskeletal elements, cell shaping and movement in the angiosperm pollen tube. J. Cell Sci. 91, 46–60
Hogan, C. (1987) Microtubule patterns during meiosis in two higher plant species. Protoplasma 138, 126–136
Kobayashi, H., Fukuda, H., Shibaoka, H. (1988) Interrelation between the spatial disposition of actin filaments and microtubules during the differentiation of tracheary elements in cultured Zinnia cells. Protoplasma 143, 29–37
Lancelle, S.A., Cresti, M., Hepler, P.K. (1987) Ultrastructure of the cytoskeleton in freeze-substituted pollen tubes of Nicotiana alata. Protoplasma 140, 141–150
Mascarenhas, J.P., Lafountain, J. (1972) Protoplasmic streaming, cytochalasin B, and growth of pollen tube. Tissue Cell 4, 11–14
Miki-Hirosige, H., Nakamura, S., Tanaka, I. (1988) Ultrastructural research on cell wall regeneration by cultured pollen protoplasts of Lilium longiflorum. Sex. Plant Reprod. 1, 36–45
Perdue, T.D., Parthasarathy, M.V. (1985) In situ localization of F-actin in pollen tubes. Eur. J. Cell Biol. 39, 13–20
Pierson, E.S. (1988) Rhodamine-phalloidin staining of F-actin in pollen after dimethylsulphoxide permeabilization. A comparison with the conventional formaldehyde preparation. Sex. Plant Reprod. 1, 83–87
Pierson, E.S., Derksen, J., Traas, J.A. (1986) Organization of microfilaments and microtubules in pollen tubes grown in vitro or vivo in various angiosperms. Eur. J. Cell Biol. 41, 14–18
Pierson, E.S., Kengen, H.M.P., Derksen, J. (1989) Microtubules and actin filaments co-localize in pollen tubes of Nicotiana tabacum L. and Lilium longiflorum Thunb. Protoplasma 150, 75–77
Raudaskoski, M., Astrom, H., Perttila, K., Virtanen, L, Louhelainen, J. (1987) Role of the microtubule cytoskeleton in pollen tubes: An immunocytochemical and ultrastructural approach. Biol. Cell 61, 177–188
Rutten, T.L.M., Derksen, J. (1990) Organization of actin filaments in regenerating and outgrowing subprotoplasts from pollen tubes of Nicotiana tabacum L. Planta 180, 471–479
Sanger, J.M., Jackson, W.T. (1971) Fine structure study of pollen development in Haemanthus katherinae Baker. II. Microtubules and elongation of generative cells. J. Cell Sci. 8, 303–315
Sheldon, J.M., Dickinson, H.G. (1986) Pollen wall formation in Lilium: The effect of chaotropic agents, and the organization of the microtubular cytoskeleton during pattern development. Planta 168, 11–23
Sheldon, J.M., Hawes, C. (1988) The actin cytoskeleton during male meiosis in Lilium. Cell Biol. Int. Rep. 12, 471–476
Tanaka, I. (1988) Isolation of generative cells and their protoplasts from pollen of Lilium longiflorum. Protoplasma 142, 68–73
Tanaka, I., Taguchi, T., Ito, M. (1979) Studies on microspore development in liliaceous plants. I. The duration of the cell cycle and developmental aspects in lily microspores. Bot. Mag. Tokyo 92, 291–298
Tanaka, I., Kitazume, C., Ito, M. (1987) The isolation and culture of lily pollen protoplasts. Plant Sci. 50, 205–211
Tanaka, I., Nakamura, S., Miki-Hirosige, H. (1989) Structural features of isolated generative cells and their protoplasts from pollen of some liliaceous plants. Gamete Res. 24, 361–374
Terasaka, O., Niitsu, T. (1990) Unequal cell division and chromatin differentiation in pollen grain cells II. Microtubule dynamics associated with the unequal cell division. Bot. Mag. Tokyo 103, 133–142
Tiwari, S.C., Polito, V.S. (1988) Spatial and temporal organization of actin during hydration, activation, and germination of pollen in Pyrus communis L.: a population study. Protoplasma 147, 5–15
Tiwari, S.C., Polito, V.S. (1990a) An analysis of the role of actin during pollen activation leading to germination in pear (Pyrus communis L.): treatment with cytochalasin D. Sex. Plant Reprod. 3, 121–129
Tiwari, S.C., Polito, V.S. (1990b) The initiation and organization of micro tubules in germinating pear (Pyrus communis L.) pollen. Eur. J. Cell Biol. 53, 384–389
Ueda, K., Miyamoto, Y., Tanaka, I. (1990) Fusion studies of pollen protoplasts and generative cell protoplasts in Lilium longiflorum. Plant Sci. 72, 259–266
Van Lammeren, A.A.M., Keijzer, C.J., Willemse, M.T.M., Kieft, H. (1985) Structure and function of the microtubular cytoskeleton during pollen development in Gasteria verrucosa (Mill.) H. Duval. Planta 165, 1–11
Van Lammeren, A.A.M., Bednara, J., Willemse, M.T.M. (1989) Organization of the actin cytoskeleton during pollen development in Gasteria verrucosa (Mill.) H. Duval visualized with rhodamine-phalloidin. Planta 178, 531–539
White, P.R. (1963) The cultivation of animal and plant cells. 2nd edn., Ronald Press, New York
Zhou, C. (1989a) A study on isolation and culture of pollen protoplasts. Plant Sci. 59, 101–108
Zhou, C. (1989b) Cell divisions in pollen protoplast culture of Hemerocallis fulva L. Plant Sci. 62, 229–235
Author information
Authors and Affiliations
Additional information
The authors are grateful to Dr. P. Lumsden (Lancashire Polytechnic, UK) for critical reading of the manuscript. This work was supported in part by Grants-in-Aid for Scientific Research (No. 01540581 and No. 02242101 to I.T.) from the Ministry of Education, Science and Culture, Japan.
Rights and permissions
About this article
Cite this article
Tanaka, I., Wakabayashi, T. Organization of the actin and microtubule cytoskeleton preceding pollen germination. Planta 186, 473–482 (1992). https://doi.org/10.1007/BF00198026
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00198026