Abstract
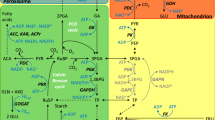
In C4 grasses belonging to the NADP-malic enzyme-type subgroup, malate is considered to be the predominant C4 acid metabolized during C4 photosynthesis, and the bundle sheath cell chloroplasts contain very little photosystem-II (PSII) activity. The present studies showed that Flaveria bidentis (L.), an NADP-malic enzyme-type C4 dicotyledon, had substantial PSII activity in bundle sheath cells and that malate and aspartate apparently contributed about equally to the transfer of CO2 to bundle sheath cells. Preparations of bundle sheath cells and chloroplasts isolated from these cells evolved O2 at rates between 1.5 and 2 μmol · min−1 · mg−1 chlorophyll (Chl) in the light in response to adding either 3-phosphoglycerate plus HCO −3 or aspartate plus 2-oxoglutarate. Rates of more than 2 μmol O2 · min−1 · mg−1 Chl were recorded for cells provided with both sets of these substrates. With bundle sheath cell preparations the maximum rates of light-dependent CO2 fixation and malate decarboxylation to pyruvate recorded were about 1.7 μmol · min−1 · mg−1 Chl. Compared with NADP-malic enzyme-type grass species, F. bidentis bundle sheath cells contained much higher activities of NADP-malate dehydrogenase and of aspartate and alanine aminotransferases. Time-course and pulse-chase studies following the kinetics of radiolabelling of the C-4 carboxyl of C4 acids from 14CO2 indicated that the photosynthetically active pool of malate was about twice the size of the aspartate pool. However, there was strong evidence for a rapid flux of carbon through both these pools. Possible routes of aspartate metabolism and the relationship between this metabolism and PSII activity in bundle sheath cells are considered.
Similar content being viewed by others
Abbreviations
- DHAP:
-
dihydroxyacetone phosphate
- NADP-ME(-type):
-
NADP-malic enzyme (type)
- NADP-MDH:
-
NADP-malate dehydrogenase
- OAA:
-
oxaloacetic acid
- 2-OG:
-
2-oxoglutarate
- PEP:
-
phosphoenolpyruvate
- PGA:
-
3-phosphoglycerate
- Pi:
-
orthophosphate
- Ru5P:
-
ribulose 5-phosphate
References
Agostino A, Furbank RT, Hatch MD (1989) Maximizing photosynthetic activity and cell integrity in isolated bundle sheath cells from C4 species. Aust J Plant Physiol 16: 279–290
Anderson JM (1986) Photoregulation of the composition, function and structure of thylakoid membranes. Annu Rev Plant Physiol 37: 93–136
Arnon DA (1949) Copper enzymes in chloroplasts. Copper enzymes in Beta vulgaris. Plant Physiol 24: 1–15
Ashton AR, Burnell JN, Furbank RT, Jenkins CLD, Hatch MD (1990) Enzymes of C4 photosynthesis. In: Lea PJ (ed) Methods in plant biochemistry, vol. 3. Academic Press, London, pp 39–72
Chapman KSR, Hatch MD (1981) Aspartate stimulation of malate decarboxylation in Zea mays bundle sheath cells: possible role in the regulation of C4 photosynthesis. Biochem Biophys Res Commun 86: 1274–1280
Chapman KSR, Berry JA, Hatch MD (1980) Photosynthetic metabolism in the bundle sheath cells of the C4 species Zea mays: sources of ATP and NADPH and the contribution of photosystem II. Arch Biochem Biophys 202: 330–341
Chitty JA, Furbank RT, Marshall JS, Chen Z, Taylor WC (1994) Genetic transformation of the C4 plant Flaveria bidentis. Plant J 6: 949–956
Edwards GE, Lilley RM, Craig S, Hatch MD (1979) Isolation of intact and functional chloroplasts from mesophyll and bundle sheath protoplasts of the C4 plant Panicum miliaceum. Plant Physiol 63: 821–827
Furbank RT, Agostino A, Hatch MD (1990) C4 acid decarboxylation and photosynthesis in the bundle sheath cells of NAD-malic enzyme-type C4 plants. Arch Biochem Biophys 276: 374–381
Gutierrez M, Huber SC, Ku SB, Kanai R, Edwards GE (1974) Intracellular location of carbon metabolism in mesophyll cells of C4 plants. In: Avron M (ed) Proc Third Int Congress of Photosynthesis. Elsevier, Amsterdam, pp 1219–1230
Hatch MD (1971) The C4 pathway of photosynthesis: evidence for an intermediate pool of CO2 and the donor C4 dicarboxylic acid. Biochem J 125: 425–432
Hatch MD (1979) Mechanism of C4 photosynthesis in Chloris gayana. Pool sizes and the kinetics of 14CO2 incorporation into 4 carbon and 3-carbon intermediates. Arch Biochem Biophys 194: 117–127
Hatch MD (1987) C4 photosynthesis: a unique blend of modified biochemistry anatomy and ultrastructure. Biochim Biophys Acta 895: 81–106
Hatch MD, Kagawa T, Craig S (1975) Subdivision of C4-pathway species based on differing C4 acid decarboxylating systems and ultrastructural features. Aust J Plant Physiol 2: 111–128
Hatch MD, Agostino A, Jenkins CLD (1995) Measurement of the leakage of CO2 from bundle sheath cells of leaves during C2 photosynthesis. Plant Physiol 108: 173–181
Jenkins CLD, Boag S (1985) Isolation of bundle sheath cell chloroplasts from the NADP-ME-type C4 plant Zea mays. Plant Physiol 79: 84–89
Ku SB, Gutierrez M, Kanai R, Edwards GE (1974) Photosynthesis in mesophyll protoplasts and bundle sheath cells of C4 plants: chlorophyll and Hill reaction studies. Z Pflanzenphysiol 72: 320–337
Leegood RC (1990) Enzymes of the Calvin cycle. In: Lea PJ (ed) Methods in plant biochemistry, vol 3. Academic Press, London, pp 15–37
Lunn JE, Hatch MD (1995) Primary partitioning and storage of photosynthate in sucrose and starch in leaves of C4 leaves. Planta 197: 385–391
MacKinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140: 315–322
Moore B, Ku MSB, Edwards GE (1984) Isolation of leaf bundle sheath protoplasts from C4 dicot species and intracellular location of selected enzymes. Plant Sci Lett 35: 127–138
Porra RJ, Thompson WA, Kreideman PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b extracted in four different solvents. Biochim Biophys Acta 975: 384–394
Repo E, Hatch MD (1976) Photosynthesis in Gomphrena celosioides and its classification amongst C4-pathway plants. Aust J Plant Physiol 3: 863–876
Woo KC, Pyliotis NA, Downton WJS (1971) Thylakoid aggregation and chlorophyll a to b ratios in C4 plants. Z Pflanzenphysiol 64: 400–413
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meister, M., Agostino, A. & Hatch, M.D. The roles of malate and aspartate in C4 photosynthetic metabolism of Flaveria bidentis (L.). Planta 199, 262–269 (1996). https://doi.org/10.1007/BF00196567
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00196567