Abstract
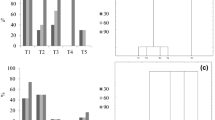
Although much information is available concerning the involvement of jasmonates in the regulation of plant development, few reports are devoted to their effects in tissue culture. In the present study, the influence of jasmonic acid (JA) on shoot and bulb formation in tissue culture of garlic (Allium sativum cv. Ptuj) was studied. Isolated basal plates were placed onto Gamborg's B5 medium. JA significantly enhanced the shoot and bulb development in concentrations from 1–10 μM. When the combination of 10 μM JA and 5 μM 2-iP was used in the initiation media, the average number of shoots was 30 after 6 weeks of culture. The bulbs formed on approximately 50% of shoots. Results described in this article show that JA might play an important role in the formation of storage organs in plants, in this case on garlic bulbs.
Similar content being viewed by others
References
Abe M, Shibaoka H, Yamane H, Takahashi N (1990) Cell cycle- dependent disruption of microtubuls by methyl jasmonate in tobacco BY-2 cells. Protoplasma 156:1–8
Anderson JM (1988) Jasmonic acid-dependent increase in the level of specific polypeptides in soybean suspension cultures and seedlings. J Plant Growth Regul 7:203–211
Anderson JM (1989) Membrane-derived fatty acids as precursors to second messengers. In: Boss WF, Morre DJ (eds) Second messengers in plant growth and development. Alan R Liss, New York, pp 181–212
Ayuso P, Pena-Iglesias A (1981) The elimination of garlic viruses by thermotherapy and/or tissue culture. Cell Biology International Reports 5:835
Bhojwani SS (1980) In vitro propagation of garlic by shoot proliferation. Sci Hort 13:47–52
Blažina I, Ravnikar M, Žolnir M, Korošec-Koruza Z, Gogala N (1991) Regeneration of GFLV-free grapevines and synchronisation of micropropagation in vitro. Acta Hort 289: 87–88
Conci VC, Nome SF (1991) Virus free garlic (Allium sativum L.) plants obtained by thermotherapy and meristem tip culture. J Phytopath 132:186–192
Farmer EE, Ryan CA (1990) Interplant communication: Airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci USA 87: 7713–7716
Farmer EE, Ryan CA (1992) Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. The Plant Cell 4:129–134
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Research 50:151–158
Gundlach H, Müller MJ, Kutchan TM, Zenk MH (1992) Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci USA 89:2389–2393
Herrmann G, Lehmann J, Peterson A, Sembdner G, Weidhase RA, Parthier B (1989) Species and tissue specificity of jasmonate-induced abundant proteins. J Plant Physiol 134:703–709
Koda Y, Kikuta Y, Tazaki H, Tsujino Y, Sakamura S, Yoshihara T (1991) Potato tuber-inducing activities of jasmonic acid and related compounds. Phytochemistry 30:1435–1438
Koda Y (1992) The role of jasmonic acid and related compounds in the regulation of plant development. International Review of Cytology 135:155–199
Leshem YY (1987) Membrane phospholipid catabolism and Ca2+ activity in control of senescence. Physiol Plantarum 69:551–559
Meyer A, Miersch O, Büttner C, Dathe W, Sembdner G, Schreiber K (1984) Occurrence of the plant growth regulator jasmonic acid in plants. Plant Growth Regul 3:1–8
Mita T, Shibaoka H (1983) Changes in microtubules in onion leaf sheath cells during bulb development. Plant Cell Physiol 24:109–117
Novak FJ (1990) Allium tissue culture In: Rabinowitch HD, Brewster JL (eds) Onions and allied crops, Vol I. CRC Press, Boca Raton, Florida, pp 233–250
Parthier B (1990) Jasmonates: Hormonal regulators or stress factors in leaf senescence? J Plant Growth Regul 9:57–63
Ravnikar M, Gogala N (1990) Regulation of potato meristem development by jasmonic acid in vitro. J Plant Growth Regul 9:233–236
Ravnikar M, Rode J, Gogala N, Benedičič D (1990) Regulation of organogenesis with jasmonic acid. Acta Hort 280:169–172
Ravnikar M, Vilhar B, Gogala N (1992) Stimulatory effects of jasmonic acid on potato stem node and protoplast culture. J Plant Growth Regul 11:29–33
Sembdner G, Herrmann G, Schliemann W (1989) Growth. Progress in Botany 51:134–164
Staswick PE (1990) Novel regulation of vegetative storage protein genes. Plant Cell 2:1–6
Staswick PE (1992) Jasmonates, genes, and fragrant signals. Plant Physiol 99:804–807
Ueda J, Kato J (1982) Inhibition of cytokinin-induced plant growth by jasmonic acid and its methyl ester. Physiol Plant 54:249–252
Van den Berg JH, Ewing EE (1991) Jasmonates and their role in plant growth and development, with special reference to the control of potato tuberization: A review. American Potato Journal 68:781–794
Van Dijk P, Verbeek M, Bos L (1991) Mite-borne virus isolates from cultivated Allium species, and their classification into two new rymoviruses in the family Potyviridae. Neth J Pl Path 97:381–399
Vilhar B, Ravnikar M, Schara M, Nemec M, Gogala N (1991) The influence of jasmonic acid on biophysical properties of potato leaf protoplasts and roots. Plant Cell Reports 10:541–544
Walkey DGA, Webb MJW, Bolland CJ, Miller A (1987) Production of virus-free garlic (Allium sativum L.) and shallot (A. Scalonicum L.). J Hort Sci 62:211–220
Weidhase RA, Lehmann J, Kramell H, Sembdner G, Parthier B (1987) Degradation of ribulose-1,5-bisphosphate carboxylase and chlorophyll in senescing barley leaf segments triggered by jasmonic acid methylester, and counteraction by cytokinin. Physiol Plantarum 69:161–166
Weidhase RA, Kramell HM, Lehmann J, Liebisch HW, Lerbs W, Parthier B (1987) Methyljasmonate-induced changes in the polypeptide pattern of senescing barley leaf segments. Plant Science 51:177–186
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ravnikar, M., Žel, J., Plaper, I. et al. Jasmonic acid stimulates shoot and bulb formation of garlic in vitro. J Plant Growth Regul 12, 73 (1993). https://doi.org/10.1007/BF00193236
Received:
Accepted:
DOI: https://doi.org/10.1007/BF00193236