Abstract
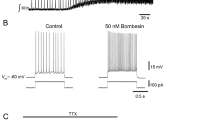
Applying the non-hydrolyzable cholinergic agonist carbachol (CCh) to the cerebral ganglion of Aplysia elicits sustained, regular bursts of activity in the buccal ganglia resembling those seen during biting. The threshold for bursting is ∼ 102−4 M. Bursting begins after a 2 to 5 min delay. The burst frequency increases over the first 5 bursts, reaching a plateau value of ∼ 3 per minute. Bursting is maintained for over 10 min. Some of the effects of CCh may be attributed to its ability to depolarize and fire CBI-2, a command-like neuron in the cerebral ganglion that initiates biting. CBI-2 is also depolarized by ACh, and by stimulating peripheral sensory nerves. Excitation of CBI-2 caused by carbachol is partially blocked by the muscarinic antagonist atropine. We examined whether CCh-induced bursting is modified in ganglia taken from Aplysia that previously experienced treatments inhibiting feeding, such as satiation, head shock contingent or non-contingent with food, and training animals with an inedible food. No treatment consistently and repeatedly affected the latency, the peak burst period, the length of time that bursting was maintained, or the threshold CCh concentration for eliciting bursting. However, there was a decrease in the rate of the buildup of the buccal ganglion program in previously satiated animals.
Similar content being viewed by others
References
Arshavsky YI, Deliagina TG, Gamkrelidze GN, Orlovsky GN, Panchin YV, Popova LB, Shupliakov OV (1993a) Pharmacologically induced elements of the hunting and feeding behavior in the pteropod mollusc Clione limacina. I. Effects of GABA. J Neurophysiol 69: 512–521
Arshavsky YI, Deliagina TG, Gamkrelidze GN, Orlovsky GN, Panchin YV, Popova LB, Shupliakov OV (1993b) Pharmacologically induced elements of the hunting and feeding behavior in the pteropod mollusc Clione limacina. II. Effects of physostigmine. J Neurophysiol 69: 523–532
Benjamin PR, Eliot CGH, Ferguson GP (1985) Neural network analysis in the snail brain. In: Selverston AI (eds) Model neural networks and behavior. Plenum Press, pp 87–108.
Blankenship JE, Wachtel H, Kandel ER (1971) Ionic mechanisms of excitatory, inhibitory, and dual synaptic actions mediated by an identified interneuron in abdominal ganglion of Aplysia. J Neurophysiol 34: 76–92
Brown DA (1988) M currents. Ion Channels 1: 55–94
Cappell MS, Spray DC, Susswein AJ, Bennett MVL (1989) Neuronal analysis of pharyngeal peristalsis in the gastropod Navanax in terms of identified motoneurons innervating identified muscle bands: II. Radial and circumferential motor fields. Brain Res 502: 266–279
Church PJ, Lloyd PE (1994) Activity of multiple identified motor neurons recorded intracellularly during evoked feeding like motor programs in Aplysia. J Neurophysiol 72: 1794–1809
Cohen JL, Weiss KR, Kupfermann I (1978) Motor control of buccal muscles in Aplysia. J Neurophysiol 41: 157–180
Delaney K, Gelperin A (1990) Cerebelar interneurons controlling fictive feeding in Limax maximus. II. Initiation and modulation of fictive feeding. J Comp Physiol A 166: 311–326
Dickinson PS, Nagy F (1988) Control of a central pattern generator by an identified neurone in Crustacea: Activation of the gastric mill motor pattern by a neurone known to modulate the pyloric rhythm. J Exp Biol 136: 53–87
Elson RC, Selverston AI (1992) Mechanisms of gastric rhythm generation in the isolated stomatogastric ganglion of spiny lobsters: Bursting pacemaker potentials, synaptic interactions, and muscarinic modulation. J Neurophysiol 68: 890–907
Gardner D (1971) Bilateral symmetry and interneuronal organization in the buccal ganglia of Aplysia. Science 173: 550–553
Gardner D, Kandel ER (1972) Diphasic postsynaptic potential: A chemical synapse capable of mediating conjoint excitation and inhibition. Science 176: 675–678
Gillette R, Kovac MP, Davis WJ (1982) Control of feeding motor output by paracerebral neurons in the brain of Pleurobranchaea californica. J Neurophysiol 47: 885–908
Gorczyca MG, Budnik V, White K, Wu CF (1991) Dual muscarinic and nicotinic action on a motor program in Drosophila. J Neurobiol 22: 391–404
Hurwitz I, Goldstein RS, Susswein AJ (1994) Compartmentalization of pattern-initiation and motor functions in the B31 and B32 neurons of the buccal ganglia of Aplysia californica. J Neurophysiol 71: 1514–1527
Hurwitz I, Neustadter D, Morton D, Chiel HJ, Susswein AJ (1996) Activity patterns of the B31/B32 pattern initiators innervating the I2 muscle of the buccal mass during normal feeding movements in Aplysia californica. J Neurophysiol 75: 1309–1326
Hurwitz I, Susswein AJ (1996) Identification and characterization of a neuron responsible for the phase switch from protraction to retraction in buccal motor programs of Aplysia californica. J Neurophysiol 75: 1327–1344
Jahan-Parwar B, Wilson AH, Fredman SM (1983) Role of proprioceptive reflexes in control of feeding muscles of Aplysia. J Neurophysiol 49: 1469–1480
Kehoe J (1990) Cyclic AMP-induced slow inward current: Its synaptic manifestation in Aplysia neurons. J Neurosci 10: 3208–3218
King MS, Delaney K, Gelperin A (1987) Acetylcholine activates cerebral interneurons and feeding motor program in Limax maximus. J Neurobiol 18: 509–530
Krnjevic K (1974) Chemical nature of synaptic transmission in vertebrates. Physiol Rev 54: 418–540
Kupfermann I (1974a) Feeding in Aplysia: A simple system for the study of motivation. Behav Biol 10: 1–26
Kupfermann I (1974b) Dissociation of the appetitive and consummatory phases of feeding behavior in Aplysia: A lesion study. Behav Biol 10: 89–97
Kupfermann I, Pinsker HM (1968) A behavioral modification of the feeding reflex in Aplysia californica. Comm Behav Biol 2: 13–17
Kupfermann I, Weiss KR (1982) Activity of an identified serotonergic neuron in free moving Aplysia correlates with behavioral arousal. Brain Res 241: 334–337
Kupfermann I, Teyke T, Rosen SC, Weiss KR (1991) Studies of behavioral state in Aplysia. Biol Bull 180: 262–268
Kuslansky B, Weiss KR, Kupfermann I (1987) Mechanisms underlying satiation of feeding behavior of the mollusc Aplysia. Behav Neural Biol 48: 278–303
Le Corronc H, Hue B (1993) Pharmacological and electrophysiological characterization of a postsynaptic muscarinic receptor in the central nervous system of the cockroach. J Exp Biol 181: 257–278
Lotshaw DP, Lloyd PE (1990) Peptidergic and serotonergic facilitation of a neuromuscular synapse in Aplysia. Brain Res 526: 81–94
Lloyd PE, Frankfurt M, Stevens P, Kupfermann I, Wess KR (1987) Biochemical and immunocytological localization of the neuropeptides FMRFamide, SCPa, SCPb, to neurons involved in the regulation of feeding in Aplysia. J Neurosci 7: 1123–1132
Marder E, Paupardin-Tritsch P (1978) The pharmacological properties of some crustacean neuronal acetylcholine, γ-aminobutyric acid and L-glutamate responses. J Physiol (Lond) 280: 213–236
Miller MW, Rosen SC, Schissel SL, Cropper EC, Kupfermann I, Weiss KR (1994) A population of SCP-containing neurons in the buccal ganglion of Aplysia are radula mechanoafferents and receive excitation of central origin. J Neurosci 14: 7008–7023
Morielli AD, Matera EM, Kovac MP, Shrum RG, McCormack KJ, Davis WJ (1986) Cholinergic suppression: A postsynaptic mechanism of long-term associative learning. Proc Natl Acad Sci USA 83: 4556–4560
Morton DW, Chiel HJ (1993a) In vivo buccal nerve activity that distinguishes ingestion from rejection can be used to predict behavioral transition in Aplysia. J Comp Physiol A 172: 17–32
Morton DW, Chiel HJ (1993b) The timing of activity in motor neurons that produce radula movements distinguishes ingestion from rejection in Aplysia. J Comp Physiol A 173: 519–536
Pinsker HM, Hening WA, Carew TJ, Kandel ER (1973) Long-term sensitization of a defensive withdrawal reflex in Aplysia. Science 182: 1039–1042
Plummer MR, Kirk MD (1990) Premotor neuron-B51 and neuronB52 in the buccal ganglia of Aplysia californica - Synaptic connections, effects on ongoing motor rhythms, and peptide modulation. J Neurophysiol 63: 539–558
Rosen SC, Teyke T, Miller MW, Weiss KR, Kupfermann I (1991a) Identification and characterization of cerebral-to-buccal interneurons implicated in the control of motor programs associated with feeding in Aplysia. J Neurosci 11: 3630–3655
Rosen SC, Weiss KR, Kupfermann I (1991b) Command of feeding in Aplysia: Implications of a network of identified interganglionic interneurons in the buccal and cerebral ganglia. Soc Neurosci Abstr 17: 1490
Quinlan EM, Gregory K, Murphy AD (1995) An identified glutaminergic interneuron patterns feeding motor activity via both excitation and inhibition. J Neurophysiol 73: 945–956
Quinlan EM, Murphy AD (1991) Glutamate as a putative transmitter in the buccal central pattern generator of Helisoma trivolvis. J Neurophysiol 66: 1264–1271
Schwarz M, Feldman E, Susswein AJ (1991) Variables affecting long-term memory of learning that a food is inedible in Aplysia. Behav Neurosci 105: 193–201
Schwarz M, Markovich S, Susswein AJ (1988) Parametric features of inhibition of feeding in Aplysia by associative learning, satiation and sustained lip stimulation. Behav Neurosci 102: 124–133
Scott ML, Govind CK, Kirk MD (1991) Neuromuscular organization of the buccal system in Aplysia californica. J Comp Neurol 312: 207–222
Scott ML, Li Y, Kirk ML (1995) Functional regeneration in the feeding system of Aplysia: Behavioral recovery correlated with changes in buccal motor output. J Neurophysiol 73: 39–55
Siegler MVS (1977) Motor neurone coordination and sensory modulation in the feeding motor system of the mollusc Pleurobranchaea. J Exp Biol 71: 27–48
Susswein AJ, Byrne JH (1988) Identification and characterization of neurons initiating patterned neural activity in the buccal ganglia of Aplysia. J Neurosci8: 2049–2061
Susswein AJ, Kupfermann I (1975) Localization of bulk stimuli underlying satiation in Aplysia. J Comp Physiol 101: 309–328
Susswein AJ, Schwarz M, Feldman E (1986) Learned changes of feeding behavior in Aplysia in response to edible and inedible foods. J Neurosci 6: 1513–1527
Susswein AJ, Weiss KR, Kupfermann I (1978) The effects of food arousal on the latency of biting in Aplysia. J Comp Physiol 123: 31–41
Susswein AJ, Rosen SC, Kupfermann I (1994) An isolated ganglia preparation for examining plasticity in the feeding system of Aplysia. Soc Neurosci Abstr 20: 580
Teyke T, Rosen SR, Weiss KR, Kupfermann I (1993) Dopaminergic neuron B20 generates rhythmic neuronal activity in the feeding motor circuitry of Aplysia. Brain Res 630: 226–237
Teyke T, Weiss KR, Kupfermann I (1990) Appetitive feeding behavior of Aplysia - behavioral and neural analysis of directed head turning. J Neurosci 10: 3922–3934
Teyke T, Weiss KR, Kupfermann I (1992) Orientation of Aplysia californica to distant food sources. J Comp Physiol A 170: 281–290
Vilim FS, Cropper EC, Rosen SC, Tenenbaum R, Kupfermann I, Weiss KR (1994) Structure, localization, and action of buccalin B: A bioactive peptide from Aplysia. Peptides 15: 959–969
Walters ET, Carew TJ, Kandel ER (1981) Associative learning in Aplysia. Evidence for conditioned fear in an invertebrate. Science 211: 504–506
Weight FF, Votava J (1970) Slow synaptic excitation in sympathetic ganglion cells: Evidence for synaptic inactivation of potassium conductance. Science 170: 755–758
Weiss KR, Cohen JL, Kupfermann I (1978) Modulatory control of buccal musculature by a serotonergic neuron (metacerebral cell) in Aplysia. J Neurophysiol 41: 181–203
Weiss KR, Kupfermann I (1976) Homology of giant serotonergic neurons (metacerebral cells) in Aplysia and pulmonate molluscs. Brain Res 117: 33–49
Wieland SJ, Gelperin A (1983) Dopamine elicits feeding motor program in Limax maximus. J Neurosci 3: 1735–1745
Yeoman MS, Parish DC, Benjamin PR (1993) A cholinergic modulatory interneuron in the feeding system of the snail, Lymnaea. J Neurophysiol 70: 37–50
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Susswein, A.J., Rosen, S.C., Gapon, S. et al. Characterization of buccal motor programs elicited by a cholinergic agonist applied to the cerebral ganglion of Aplysia californica . J Comp Physiol A 179, 509–524 (1996). https://doi.org/10.1007/BF00192317
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00192317