Abstract
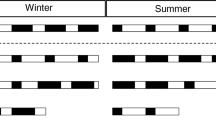
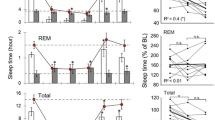
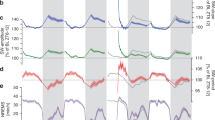
To asses the influence of photoperiod on sleep regulation EEG, EMG, and cortical temperature were continuously recorded for two baseline days and after 4 h sleep deprivation in Djungarian hamsters (Phodopus sungorus) adapted to a short photoperiod (light dark 8∶16). Comparison to previous data collected in a long photoperiod (light∶dark 16∶8) showed several major effects of photoperiod: 1. A prominent change in the 24-h distribution, duration and number of vigilance state episodes, whereas the total amount of sleep and waking was unchanged; 2. Cortical temperature was 0.7°C lower in the short photoperiod; 3. There was a significant negative correlation between cortical temperature and the frequency of REM sleep episodes; and 4. Absolute EEG power density showed a marked reduction in the short photoperiod. After sleep deprivation EEG slow-wave activity (mean power density 0.75–4.0 Hz) in NREM sleep showed a remarkably similar increase in both photoperiods demonstrating the robustness of the homeostatic regulation of sleep. Cortical temperature remained above baseline values after sleep deprivation in the short photoperiod whereas a negative rebound was present in the long photoperiod. Our results support the hypothesis that cortical temperature has a strong influence on REM sleep propensity and indicate the possibility of an optimum cortical temperature for recovery sleep after sleep deprivation. The lower EEG power density in the short photoperiod may contribute to energy conservation.
Similar content being viewed by others
Abbreviations
- LP :
-
long photoperiod
- NREM :
-
non-rapid-eye-movement
- REM :
-
rapid-eye-movement
- SCN :
-
suprachiasmatic nucleus
- SD :
-
sleep deprivation
- SP :
-
short photoperiod
- SWA :
-
slow-wave activity
- T CRT :
-
cortical temperature
References
Arendt J, Symons AM, Laud C (1981) Pineal function and photoperiod in the ewe. In: Ortovant R, Pelletier J, Ravault J-P (eds) Photoperiodism and reproduction in invertebrates. INRA Publications, Nouzilly, pp 219–229
Aschoff J (1962) Spontane lokomotorische Aktivität. In: Helmcke JG, Von Lengerken H, Stark D (eds) Handbuch der Zoologie. Vol 8. Part 2. De Gruyter, Berlin, pp 2–76
Aschoff J (1993) On the relationship between motor activity and the sleep-wake cycle in humans during temporal isolation. J Biol Rhythms 8: 33–46
Aschoff J, Meyer-Lohmann J (1955) Die Aktivitätsperiodik von Nagern im künstlichen 24-Stunden-Tag mit 6–20 Stunden Lichtzeit. Z Vergl Physiol 37: 107–117
Bartness TJ, Goldman BD (1989) Mammalian pineal melatonin: A clock for all seasons. Experientia 45: 939–945
Benington JH, and HC Heller (1994) Does the function of REM sleep concern non-REM sleep or waking? Prog Neurobiol 44: 433–449
Bittman EL, Bartness TJ, Goldman BD, DeVries GJ (1991) Suprachiasmatic and paraventricular control of photoperiodism in Siberian hamsters. Am J Physiol 260: R90-R101
Borbély AA (1982) A two-process model of sleep regulation. Human Neurobiol 1: 195–204
Borbély AA, Neuhaus HU (1978) Daily pattern of sleep, motor activity, and feeding in the rat: effects of regular and gradually extended photoperiods. J Comp Physiol 124: 1–14
Borbély AA, Neuhaus HU (1979) Sleep-deprivation: effects on sleep and EEG in the rat. J Comp Physiol 133: 71–87
Borbély AA, Baumann F, Brandeis BD, Strauch I, Lehmann D (1981) Sleep deprivation, effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol 51: 438–493
Borbély AA, Tobler I, Hanagasioglu M (1984) Effects of sleep deprivation on sleep and EEG power spectra in the rat. Behav Brain Res 14: 171–182
Buresová M, Dvoráková M, Zvolsky P, Illnerová H (1992) Human circadian rhythm in serum melatonin in short winter days and in simulated artificial long days. Neurosci Lett 136: 173–176
Cabral R, Prior PF, Scott DF, Brierley JB (1977) Reversible profound depression of cerebral cortical activity in hyperthermia. Electroencephalogr Clin Neurophysiol 42: 697–701
Daan S, Beersma DGM, Borbély AA (1984) Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol246: R161-R178
Deboer T, Tobler I (1994) Sleep EEG after daily torpor in the Djungarian hamster: Similarity to the effects of sleep deprivation. Neurosci Lett 166: 35–38
Deboer T, Tobler I (1995) Temperature dependence of EEG frequencies during natural hypothermia. Brain Res 670: 153–156
Deboer T, Franken P, Tobler I (1994) Sleep and cortical temperature in the Djungarian hamster under baseline conditions and after sleep deprivation. J Comp Physiol A 174: 145–155
Dijk DJ, Daan S (1989) Sleep EEG spectral analysis in a diurnal rodent: Eutamias sibiricus. J Comp Physiol A 165: 205–215
Dijk DJ, Beersma DGM, Daan S (1987) EEG power density during nap sleep: reflection of an hourglass measuring the duration of wakefulness. J Biol Rhythms 3: 207–219
Dijk DJ, Brunner DP, Borbély AA (1990) Time course of EEG power density during long sleep in humans. Am J Physiol 27: 650–661
Elliot JA, Bartness TJ, Goldman BD (1987) Role of short photoperiod and cold exposure in regulating daily torpor in Djungarian hamsters. J Comp Physiol A 161: 245–253
Figala J, Hoffmann K, Goldau G (1973)Zur Jahresperiodik beim Dsungarischen Zwerghamster Phodopus sungorus Pallas. Oecologia 12: 89–118
Franken P, Dijk DJ, Tobler I, Borbély AA (1991a) Sleep deprivation in the rat: effects on electroencephalogram power spectra, vigilance states, and cortical temperature. Am J Physiol 261:R198-R208
Franken P, Tobler I, Borbély AA (1991b) Sleep homeostasis in the rat: simulations of the time course of EEG slow-wave activity. Neurosci Lett 130: 141–144
Franken P, Tobler I, Borbély AA (1992a) Cortical temperature and EEG slow-wave activity in the rat: analysis of vigilance state related changes. Pflügers Archs 420: 500–507
Franken P, Tobler I, Borbély AA (1992b) Sleep and waking have a major effect on the 24-hr rhythm of cortical temperature in the rat. J Biol Rhythms 7: 341–352
Franken P, Tobler I, Borbély AA (1995) Long and short photoperiod in the rat; a profound effect on the 24-h pattern of sleep and cortical temperature but no effect on sleep homeostasis. Am J Physiol 269: R691-R701
Friedman L, Bergmann BM, Rechtschaffen A (1979) Effects of sleep deprivation on sleepiness, sleep intensity, and subsequent sleep in the rat. Sleep 1: 369–391
Gold S, Cahani M, Sohmer H, Horowitz M, Shahar A (1985) Effects of body temperature elevation on auditory nerve-brain-stem evoked responses and EEGs in rats. Electroencephalogr Clin Neurophysiol 60: 146–153
Harris DV, Walker JM, Berger RJ (1984) A continuum of slow-wave sleep and shallow torpor in the pocket mouse Perognatus longimembris. Physiol Zool 57: 428–434
Heldmaier G, Steinlechner S (1981a) Seasonal control of energy requirements for thermoregulation in the Djungarian hamster (Phodopus sungorus), living in natural photoperiod. J Comp Physiol 142: 429–437
Heldmaier G, Steinlechner S (1981b) Seasonal pattern and energetics of short daily torpor in the Djungarian hamster, Phodopus sungorus. Oecologia 48: 265–270
Heldmaier G, Steinlechner S, Rafael J, Vsiansky P (1981) Photoperiodic control and effects of melatonin on nonshivering thermogenesis and brown adipose tissue. Science 212: 917–919
Heldmaier G, Steinlechner S, Rafael J, Latteier B (1982) Photoperiod and ambient temperature as environmental cues for seasonal thermogenic adaptation in the Djungarian hamster, Phodopus sungorus. Int J Biometeor 26: 339–345
Heldmaier G, Steinlechner S, Ruf T, Wiesinger H, Klingenspor M (1989) Photoperiod and thermoregulation in vertebrates: body temperature rhythms and thermogenic acclimation. J Biol Rhythms 4: 251–265
Hoffmann K (1973) The influence of photoperiod and melatonin on testis size, body weight, and pelage color in the Djungarian hamster (Phodopus sungorus). J Comp Physiol 85: 267–282
Hofman MA, Swaab DF (1992) Seasonal changes in the suprachiasmatic nucleus of man. Neurosci Lett 139: 257–260
Hofman MA, Swaab DF (1993) Diurnal and seasonal rhythms of neuronal activity in the suprachiasmatic nucleus of humans. J Biol Rhythms 8: 283–295
Humlová M, Illnerová H (1992) Entrainment of the rat circadian clock controlling the pineal N-acetyltransferase rhythm depends on photoperiod. Brain Res 584: 226–236
Illnerová H (1988) Entrainment of mammalian circadian rhythms in melatonin production by light. Pineal Res Rev 6: 173–217
Illnerová H, Hoffmann K, Vaněček (1984) Adjustment of pineal melatonin and N-acteyltransferase rhythms to change from long to short photoperiod in the Djungarian hamster Phodopus sungorus. Neuroendocrinology 38: 226–231
Jouvet M, Buda C, Sastre J (1989) Increase of paradoxical sleep during hypothermia in pontine cats. In: Horne J (ed) Sleep '88.Gustav Fischer, Stuttgart, pp 85–90
Kaminski D, Weiner N, Sturm G, Wesemann W (1993) Modulation of serotonin binding sites in the brain of the Djungarian hamster, Phodopus sungorus, during adaptation to a short photoperiod. J Neural Transm 92: 159–171
Kliman RM, Lynch GR (1992) Evidence for genetic variation in the occurrence of the photoresponse of the Djungarian hamster, Phodopus sungorus. J Biol Rhythms 7: 161–173
Krilowicz BL, Glotzbach SF, Heller HC (1988) Neuronal activity during sleep and complete bouts of hibernation. Am J Physiol 255: R1008-R1019
Marks T, Schulze-Bonhage A, Wittkowski W (1993) Photoperioddependent changes in exocytotic activity in the hypophyseal pars tubularis of the Djungarian hamster, Phodopus sungorus. Cell Tissue Res 273: 287–291
Mathews CD, Guerin MV, Wang X (1991) Human plasma melatonin and urinary 6-sulphatoxy melatonin: studies in natural annual photoperiod and in extended darkness. Clin Endocrin 35: 21–27
Milette JH, Turek FW (1986) Circadian and photoperiodic effects of brief light pulses in male Djungarian hamsters. Biol Reprod 35: 327–335
Neckelmann D, Ursin R (1993) Sleep stages and EEG power spectrum in relation to acoustical stimulus arousal threshold in the rat. Sleep 16: 467–477
Parmeggiani PL, Agnati LF, Zamboni G, Cianci T (1975) Hypothalamic temperature during the sleep cycle at different ambient temperatures. Electroencephalogr Clin Neurophysiol 38: 589–596
Pittendrigh CS, Daan S (1976a) A functional analysis of circadian pacemakers in nocturnal rodents IV. Entrainment: pacemaker as clock. J Comp Physiol 106: 291–331
Pittendrigh CS, Daan S (1976b) A functional analysis of circadian pacemakers in nocturnal rodents V. Pacemaker structure: A clock for all seasons. J Comp Physiol 106: 333–355
Pittendrigh CS, Minis DH (1964) The entrainment of circadian oscillators by light and their role as photoperiodic clocks. Am Nat 98: 261–294
Reiter RJ (1993) The melatonin rhythm: Both a clock and a calendar. Experientia 49: 654–664
Reuss S, Bürger K (1994) Substance P-like immunoreactivity in the hypothalamic suprachiasmatic nucleus of Phodopus sungorus- relation to daytime, photoperiod, sex and age. Brain Res 638: 189–195
Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, Mueller PS, Newsome DA, Wehr TA (1984) Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry 41: 72–80
Ruf T, Steinlechner S, Heldmaier G (1987) Influence of photoperiod on body temperature rhythms in the Djungarian hamster. In: Hildebrandt G, Moog R, Raschke F (eds) Chronobiology & Chronomedicine basic research and applications. Peter Lang, Frankfurt a.M., pp 79 84
Steinlechner S, Heldmaier G, Weber C, Ruf T (1986) Role of photoperiod: Pineal gland interaction in torpor control. In: Heller HC, Musacchia XJ, Wang LCH (eds) Living in the cold. Elsevier, New York, pp 301–307
Tamarkin L, Baird CJ, Almeida OFX (1985) Melatonin: A coordinating signal for mammalian reproduction? Science 227:714–720
Tobler I (1992) Behavioral sleep in the Asian elephant in captivity. Sleep 15: 1–12
Tobler I, Borbély AA (1986) Sleep EEG in the rat as a function of prior waking. Electroencephalogr Clin Neurophysiol 64: 74–76
Tobler I, Franken P (1993) Sleep homeostasis in the guinea pig: similar response to sleep deprivation in the light and dark period. Neurosci Lett 164: 105–108
Tobler I, Jaggi K (1987) Sleep and EEG spectra in the Syrian hamster (Mesocricetus auratus) under baseline conditions and following sleep deprivation. J Comp Physiol A 161: 449–459
Trachsel L, Tobler I, Borbély AA (1986) Sleep regulation in rats: effects of sleep deprivation, light, and circadian phase. Am J Physiol 251: R1037-R1044
Walker JM, Garber A, Berger RJ, Heller HC (1979) Sleep and estivation (shallow torpor): continuous processes of energy conservation. Science 204: 1098–1100
Walker JM, Glotzbach SF, Berger RJ, Heller HC (1977) Sleep and hibernation in ground squirrels (Citellus spp.): electrophysiological observations. Am J Physiol 233: R213-R221
Wehr TA (1991) The duration of human melatonin secretion and sleep respond to changes in daylength (photoperiod). J Clin Endocrin Metabol 73: 1276–1280
Wehr TA, Moul WD, Barbato G, Giesen HA, Seidel JA, Barker C, Bender C (1993) Conservation of photoperiod-responsive mechanisms in humans. Am J Physiol 265: R846-R857
Wirz-Justice A, Wever RA, Aschoff J (1984) Seasonality in freerunning circadian rhythms in man. Naturwissenschaften 71: 316–319
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Deboer, T., Tobler, I. Shortening of the photoperiod affects sleep distribution, EEG and cortical temperature in the Djungarian hamster. J Comp Physiol A 179, 483–492 (1996). https://doi.org/10.1007/BF00192315
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00192315