Summary
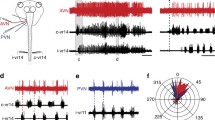
The concept of coded ‘command releasing systems’ proposes that visually specialized descending tectal (and pretectal) neurons converge on motor pattern generating medullary circuits and release — in goal-specific combination — specific action patterns. Extracellular recordings from medullary neurons of the medial reticular formation of the awake immobilized toad in response to moving visual stimuli revealed the following main results. (i) Properties of medullary neurons were distinguished by location, shape, and size of visual receptive fields (ranging from relatively small to wide), by trigger features of various moving configural stimulus objects (including preyand predator-selective properties), by tactile sensitivity, and by firing pattern characteristics (sluggish, tonic, warming-up, and cyclic). (ii) Visual receptive fields of medullary neurons and their responses to moving configural objects suggest converging inputs of tectal (and pretectal) descending neurons. (iii) In contrast to tectal monocular ‘small-field’ neurons, the excitatory visual receptive fields of comparable medullary neurons were larger, ellipsoidally shaped, mostly oriented horizontally, and not topographically mapped in an obvious fashion. Furthermore, configural feature discrimination was sharper. (iv) The observation of multiple properties in most medullary neurons (partly showing combined visual and cutaneous sensitivities) suggests integration of various inputs by these cells, and this is in principle consistent with the concept of command releasing systems. (v) There is evidence for reciprocal tectal/medullary excitatory pathways suitable for premotor warming-up. (vi) Cyclic bursting of many neurons, spontaneously or as a post-stimulus sustaining event, points to a medullary premotor/motor property.
Similar content being viewed by others
Abbreviations
- BMC :
-
branchiomotor column
- ERF :
-
excitatory receptive field
- RetF :
-
medullary (bulbar) reticular formation
- M1-12 :
-
response properties of medullary neurons
- T1-9 :
-
classes of tectal neurons
- TH1-9 :
-
classes of thalamic pretectal neurons
- tr.tb.d. :
-
tractus tectobulbaris directus
- tr.tbs.c. :
-
tractus tectobulbaris et spinalis cruciatus
- HRP :
-
horseradish peroxidase
References
Ariens Kappers CU, Huber CG, Crosby EC (1936) The comparative anatomy of the nervous system of vertebrates, including man, Vol. 1. Macmillan, New York
Chung S-H, Raymond SA, Lettvin JY (1970) Multiple meaning in single visual units. Brain Behav Evol 3:72–101
Comer CM (1987) Sensorimotor functions: what is a command, that a code may yield it? A commentary. Behav Brain Sci 10:372
Donkelaar, ten H-J, de Boer-van Juizen R, Schuoten FTM, Eggen SJH (1981) Cells of origin of descending pathways to the spinal cord in the clawed toad Xenopus laevis. Neurosci 16:2297–2312
Ebbesson SOE (1976) Morphology of the spinal cord. In: Llinás L, Precht W (eds) Frog neurobiology. Springer, Berlin Heidelberg New York, pp 679–706
Edinger L (1908) Vorlesungen über den Bau der nervösen Zentralorgane des Menschen und der Tiere, Vol. 2. Vogel, Leipzig
Ewert J-P (1984) Tectal mechanisms that underlie prey-catching and avoidance behaviors in toads. In: Vanegas H (ed) Comparative neurology of the optic tectum. Plenum Press, New York, pp 247–416
Ewert J-P (1987) Neuroethology of releasing mechanisms: preycatching in toads. Behav Brain Sci 10:337–405
Ewert J-P, Schürg-Pfeiffer E, Weerasuriya A (1984) Neurophysiological data regarding motor pattern generation in the medulla oblongata of toads. Naturwissenschaften 71:590–591
Fuller JH, Schlag JD (1976) Determination of antidromic excitation by the collision test: problems of interpretation. Brain Res 112:283–298
Gaupp E (1896) A. Ecker's and R. Wiedersheim's Anatomie des Frosches. F Viehweg & Sohn, Braunschweig
Gillette R, Kovac MP, Davis WJ (1978) Command neurons in Pleurobranchaea receive synaptic feedback from the motor network they excite. Science 199:798–801
Grobstein P (1989) Organization in the sensorimotor interface: a case study with increased resolution. In: Ewert J-P, Arbib MA (eds) Visuomotor coordination: amphibians, comparisons, models, and robots. Plenum Press, New York, pp 537–568
Grobstein P, Comer C, Kostyk SK (1983) Frog prey-catching behavior: between sensory maps and directed motor output. In: Ewert J-P, Ingle DJ, Capranica RR (eds) Advances in vertebrate neuroethology. Plenum Press, New York, pp 331–347
Grüsser O-J, Grüsser-Cornehls U (1976) Neurophysiology of the anuran visual system. In: Llinás L, Precht W (eds) Frog neurobiology. Springer, Berlin Heidelberg New York, pp 297–385
Herrick CJ (1930) The medulla oblongata of Necturus. J Comp Neurol 50:1–96
Hinde RA (1954) Changes in responsiveness to a constant stimulus. Behaviour 2:41–54
Ingle DJ (1976) Spatial vision in anurans. In: Fite KV (ed) The amphibian visual system: a multidisciplinary approach. Academic Press, New York, pp 119–140
Ingle DJ (1983) Brain mechanisms of visual localization by frogs and toads. In: Ewert J-P, Capranica RR, Ingle DJ (eds) Advances in vertebrate neuroethology. Plenum-Press, New York, pp 177–226
Julesz B (1976) Experiments in the visual perception of texture. In: Held R, Richards W (eds) Recent progress in perception. Freeman, San Francisco, pp 37–46
Kostyk SK, Grobstein P (1987a) Neuronal organization underlying visually elicited prey orienting in the frog. II. Anatomical studies on the laterality of central projections. Neurosci 21:57–82
Kostyk SK, Grobstein P (1987b) Neuronal organization underlying visually elicited prey orienting in the frog. III. Evidence for the existence of an uncrossed descending tectofugal pathway. Neurosci 21:83–96
Lázár G (1989) Cellular architecture and connectivity of the frog's optic tectum. In: Ewert J-P, Arbib MA (eds) Visuomotor coordination: amphibians, comparisons, models, and robots. Plenum Press, New York, pp 175–199
Lázár G, Tóth P, Csank G, Kicliter E (1983) Morphology and location of tectal projection neurons in frogs: a study with HRP and cobalt-filling. J Comp Neurol 215:108–120
Lettvin JY, Maturana HR, Pitts WH, McCulloch (1961) Two remarks on the visual system of the frog. In: Rosenblith WA (ed) Sensory communication. MIT Press, Cambridge, Mass, pp 757–776
Lipski J (1981) Antidromic activation of neurons as an analytic tool in the study of the central nervous system. J Neurosci Methods 4:1–32
Masino T, Grobstein P (1989a) The organization of descending tectofugal pathways underlying orienting in the frog, Rana pipiens. I. Lateralization, parcellation, and an intermediate spatial representation. Exp Brain Res 75:227–244
Masino T, Grobstein P (1989b) The organization of descending tectofugal pathways underlying orienting in the frog, Rana pipiens. II. Evidence for the involvement of a tecto-tegmentospinal pathway. Exp Brain Res 75:245–264
Masino T, Grobstein P (1990) Tectal connectivity in the frog Rana pipiens: Tectotegmental projections and a general analysis of topographic organization. J Comp Neurol 291:103–127
Matsushima T, Satou M, Ueda K (1986) Glossopharyngeal and tectal influences on tongue muscle motoneurons in the Japanese toad. Brain Res 265:198–203
Matsushima T, Satou M, Ueda K (1989) Medullary reticular neurons in the Japanese toad: morphology and excitatory inputs from the optic tectum. J Comp Physiol A 166:7–22
Mittelstaedt H, Eggert T (1989) How to transform topographically ordered spatial information into motor commands. In: Ewert J-P, Arbib MA (eds) Visuomotor coordination: Amphibians, comparisons, models, and robots. Plenum Press, New York, pp 569–585
Nieuwenhuys R, Opdam P (1976) Structure of the brain stem. In: Llinás L, Precht W (eds) Frog neurobiology. Springer, Berlin Heidelberg New York, pp 811–855
Röthig P (1927) Beiträge zum Studium des Zentralnervensystems der Wirbeltiere. 11. Über die Faserzüge im Mittelhirn, Kleinhirn und der Medulla oblongata der Urodelen und Anuren. Z Mikr Anat Forsch 10:381–472
Rubinson E (1968) Projections of the tectum opticum of the frog. Brain Behav Evol 1:529–561
Satou M, Ewert J-P (1985) The antidromic activation of tectal neurons by electrical stimuli applied to the caudal medulla oblongata in the toad Bufo bufo L. J Comp Physiol A 157:739–748
Satou M, Matsushima T, Takeuchi H, Ueda K (1985) Tongue-muscle-controlling motoneurons in the Japanese toad: topography, morphology and neuronal pathways from the ‘snappinge-voking area’ in the optic tectum. J Comp Physiol A 157:717–737
Schlag JD (1978) Electrophysiological mapping techniques. In: Robertson RT (ed) Neuroanatomical research techniques. Academic Press, New York, pp 385–404
Schlarb EM (1990) Untersuchungen über die Afferenzen der medullären medialen Formatio reticularis unter besonderer Berücksichtigung visueller Informationen. Dissertation, FB 19, Univ Kassel
Schürg-Pfeiffer E (1989) Behavior-correlated properties of tectal neurons in freely moving toads. In: Ewert J-P, Arbib MA (eds) Visuomotor coordination: amphibians, comparisons, models, and robots. Plenum Press, New York, pp 451–480
Schürg-Pfeiffer E, Ewert J-P (1981) Investigation of neurons involved in the analysis of Gestalt prey features in the frog Rana temporaria. J Comp Physiol 141:139–158
Schwippert WW, Beneke TW, Ewert J-P (1990) Responses of medullary neurons to moving visual stimuli in the common toad. II. An intracellular recording and cobalt-lysine labeling study. J Comp Physiol A 167:509–520
Tsai HJ, Ewert J-P (1988) Influence of stationary and moving background structures on the response of visual neurons in toads (Bufo bufo). Brain Behav Evol 32:27–38
Tuge H (1932) Somatic motor mechanisms in the midbrain and medulla oblongata of Chrysemys elegans (Wied). J Comp Neurol 55:185–271
Weerasuriya A (1989) In search of the motor pattern generator for snapping in toads. In: Ewert J-P, Arbib MA (eds) Visuomotor coordination: amphibians, comparisons, models, and robots. Plenum Press, New York, pp 589–614
Weerasuriya A, Ewert J-P (1981) Prey-selective neurons in the toad's optic tectum and sensorimotor interfacing: HRP studies and recording experiments. J Comp Physiol 144:429–434
Yerkes RM (1903) The instincts, habits, and reactions of the frog. Harvard Psychol Studies 1:579–638
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ewert, J.P., Framing, E.M., Schürg-Pfeiffer, E. et al. Responses of medullary neurons to moving visual stimuli in the common toad. J Comp Physiol A 167, 495–508 (1990). https://doi.org/10.1007/BF00190820
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00190820