Abstract
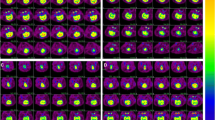
Positron emission tomography (PET) permits the study of cerebral metabolism in vivo. We performed repeated PET studies with fluorine-18 fluorodeoxyglucose (FDG) as a tracer to measure cerebral glucose metabolism for estimation of neurological prognosis in infants with suspected hypoxic-ischaemic brain injury. Fourteen infants (gestational age 35.3 ± 4.67 weeks) were examined during the neonatal period (at age 38.4±2.7 weeks) and again at the age of 3.5±0.7 months; one further infant was studied only once at the age of 2.5 months. All children also underwent ultrasound examinations. Electroencephalography and computed tomography or magnetic resonance imaging were performed according to their clinical condition and their neurological development has been followed. FDG accumulated most actively in the subcortical areas (thalami, brainstem and cerebellum) and the sensorimotor cortex during the neonatal period. The repeated PET study showed that the uptake of FDG was markedly high and increased in all brain sections of infants with normal development (n=11), whereas those with delayed development (n=4) had significantly lower values (P≤0.005).
Similar content being viewed by others
References
Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 1976;33:696–705.
Fenichel GM. Hypoxic-ischemic encephalopathy in the newborn. Arch Neurol 1983;40:261–266.
Volpe JJ. Neurology of the newborn, 2nd edn. Philadelphia: W. B. Saunders, 1987.
Chugani HT, Phelps ME. Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science 1986;231:840–843.
Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol 1987;22:487–497.
Doyle LW, Nahmias C, Firnau G, et al. Regional cerebral glucose metabolism of newborn infants measured by positron emission tomography. Dev Med Child Neurol 1983;25:143–151.
Thorp PS, Levin SD, Garnett ES, et al. Patterns of cerebral glucose metabolism using 18FDG and positron emission tomography in the neurologic investigation of the full term newborn infant. Neuropediatrics 1988;19:146–153.
Volpe JJ, Herscovitch P, Perlman JM, Raichle ME. Positron emission tomography in the newborn: extensive impairment of regional cerebral blood flow with intraventricular hemorrhage and hemorrhagic intracerebral involvement. Pediatrics 1983;72:589–601.
Volpe JJ, Herscovitch P, Perlman JM, Kreusser KL, Raichle ME. Positron emission tomography in the asphyxiated term newborn: parasagittal impairment of cerebral blood flow. Ann Neurol 1985;17:287–296.
Perlman JM, Herscovitch P, Kreusser KL, et al. Positron emission tomography in the newborn: effect of seizure on regional cerebral blood flow in an asphyxiated infant. Neurology 1985;35:244–247.
Hope PL, Costello AMdeL, Cady EB, et al. Cerebral energy metabolism studied with phosphorus NMR spectroscopy in normal and birth-asphyxiated infants. Lancet 1984;11:366–370.
Azzopardi D, Wyatt S, Cady EB, et al. Prognosis of newborn infants with hypoxic-ischemic brain injury assessed by phosphorus magnetic resonance spectroscopy. Pediatr Res 1989;25:445–451.
Ruth V, Fyhrquist F, Clemons G, Raivio KO. Cord plasma vasopressin, erythropoietin, and hypoxanthine as indices of asphyxia at birth. Pediatr Res 1988;24:490–494.
Ruth V, Autti-Rämö I, Granström M-L, Korkman M, Raivio KO. Prediction of perinatal brain damage by cord plasma vasopressin, erythropoietin, and hypoxanthine. J Pediatr 1990;116:950–954.
Ruth V. Prognostic value of creatine kinase BB-isoenzyme in high-risk newborn infants. Arch Dis Child 1989;64:563–568.
Finer NN, Robertson CM, Richards RT, et al. Hypoxicischemic encephalopathy in term neonates: perinatal factors and outcome. J Pediatr 1981;98:112–117.
Dubowitz LMS, Palmer PG, Miller G, et al. Correlation of neurologic assessment in the preterm newborn infant with outcome at 1 year. J Pediatr 1984;105:452–456.
Levene MI, Sands C, Grindulis H, Moore JR. Comparison of two methods of predicting outcome in perinatal asphyxia. Lancet 1986;1:67–69.
Calame A, Fawer CL, Anderegg A, Perentes E. Interaction between perinatal brain damage and processes or normal brain development: ultrasonographic and neurodevelopmental study in the first year of life. Dev Neurosci 1985;7:1–11.
Adsett DB, Fitz CR, Hill A. Hypoxic-ischemic cerebral injury in the term newborn: correlation of CT findings with neurological outcome. Dev Child Neurol 1985;27:155–160.
Fitzharding PM, Flodmark O, Fitz CR, Ashby S. The prognostic value of computed tomography as an adjunct to assessment of the term infant with postasphyxial encephalopathy. J Pediatr 1981;99:777–781.
Steinlin M, Dirr R, Martin E, et al. MRI following severe perinatal asphyxia: preliminary experience. Pediatr Neurol 1991;7:164–170.
Spinks TJ, Jones T, Gilardi MC, Heather JD. Physical performance of the latest generation of commercial positron scanner. IEEE Trans Nucl Sci 1988;35:721–725.
Haaparanta M, Bergman J, Solin O, Roeda D. A remotely controlled system for the routine synthesis of 18F-2-fluoro-2-deoxy-d-glucose. Nuclearmedizin (Suppl) 1984;21:823–826.
Ruotsalainen U, Eronen E, Suhonen-Polvi H, Kinnala A, Teräs M, Wegelius U. Dosimetric measurements in [18F]FDG brain studies of newborn infants. In: Voipio-Pulkki L-M, Wegelius U, eds. Medical application of cyclotrons VI. Ann Univ Turkuensis 1992;88:A75–A76.
Oldendorf WH. Expression of tissue isotope distribution. J Nucl Med 1975;16:958–959.
Woodard HQ, Bigler RB, Freed B, Russ G. Expression of tissue isotope distribution. J Nucl Med 1974;16:958–959.
Lindholm P, Minn H, Leskinen-Kallio S, Bergman J, Ruotsalainen U, Joensuu H. Influence of the blood glucose concentration on FDG uptake in cancer — a PET study. J Nucl Med 1993;34:1–6.
Kinnala A, Nuutila P, Ruotsalainen U, et al. Neonatal hypoglycaemia effects on cerebral glucose utilization in newborn infants: a [18F]FDG positron emission tomography (PET) study. In: Voipio-Pulkki L-M, Wegelius U, eds. Medical application of cyclotrons VI. Ann Univ Turkuensis 1992:88:A77–A78.
Suhonen-Polvi H, Ruotsalainen U, Kero P, et al. Functional development of the brain during the first months of life: a [18F]FDG positron emission tomography study. Fetal Diagn Ther 1992;7:85.
Greisen G. Effect of cerebral blood flow and cerebrovascular autoregulation on the distribution, type and extent of cerebral injury. Brain Pathol 1992;2:223–228.
Vannucci RC. Cerebral carbohydrate and energy metabolism in perinatal hypoxic-ischemic brain damage. Brain Pathol 1992;2:229–234.
Chugani HT, Hovda DA, Villablanca JR, Villablanca JR, Phelps ME, Xu W-F. Metabolic maturation of the brain: a study of local cerebral utilization in the developing cat. J Cereb Blood Flow Metab 1991;11:35–47.
Rubinstein M, Denays R, Ham HR, et al. Functional imaging of brain maturation in humans using iodine-123 iodoamphetamine and SPECT. J Nucl Med 1989;30:1982–1985.
Denays R, Ham H, Tondeur M, Piepsz A, Noel P. Detection of bilateral and symmetrical anomalies in technetium-99m-HMPAO brain SPECT studies. J Nucl Med 1992;33:485–490.
Chiron C, Raynaud C, Maziere B, et al. Changes in regional cerebral blood flow during brain maturation in children and adolescents. J Nucl Med 1992;33:696–703.
Kraus H, Schlenker S, Schwedesky D. Developmental changes of cerebral ketone body utilization in human infants. Hoppe Seylers Z Physiol Chem 1974;355:164–170.
Blomstrand S, Karlsson K, Kjellmer I. Measurement of cerebral blood flow in the fetal lamb with a note on the flow-distribution. Acta Physiol Scand 1978;103:1–8.
Author information
Authors and Affiliations
Additional information
Correspondence to: H. Suhonen-Polvi
Rights and permissions
About this article
Cite this article
Suhonen-Polvi, H., Kero, P., Korvenranta, H. et al. Repeated fluorodeoxyglucose positron emission tomography of the brain in infants with suspected hypoxic-ischaemic brain injury. Eur J Nucl Med 20, 759–765 (1993). https://doi.org/10.1007/BF00180905
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00180905