Summary
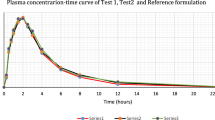
Allopurinol is converted almost completely into a single active metabolite, oxipurinol, which has the same therapeutic pattern but a much longer elimination half-life than the parent compound. Therefore both allopurinol and oxipurinol were evaluated in our bioequivalence study in healthy volunteers comparing two allopurinol brands. Bioequivalence determination was based on the 90% confidence intervals (CI) of the area under the plasma concentration time curve from time zero to infinity (AUC0−∞), of the area from time zero to the last measurable plasma concentration (AUC0−t (last)), and C max. Because of the lack of compound-specific criteria we used conventional limits for the bioequivalence range. Under these conditions the brand chosen as test preparation was judged to be bioequivalent to the reference form with respect to the extent of bioavailability, AUC0−∞, and AUC0−(last) of the parent drug. The CI of C max of allopurinol slightly exceeded the upper limit of 130%, so that bioequivalence was not confirmed with regard to the rate of bioavailability of the parent compound. The CI values of both AUC and C max of the active metabolite were tighter than those of allopurinol. In addition, the CI values of C max of oxipurinol were smaller than those of the corresponding AUC. As a consequence the test drug can clearly be accepted as bioequivalent, based on metabolite data. Since the active metabolite is of greater therapeutic significance than the parent drug, assessment of the bioequivalence of allopurinol preparations needs to be based on oxipurinol rather than allopurinol. Our data provide further evidence that establishing compound-specific criteria is required for bioequivalence evaluation in drugs with a single active metabolite.
Similar content being viewed by others
Abbreviations
- AUC0−1 (last) :
-
area under the plasma concentration time curve from time zero to the last measurable plasma concentration
- AUC0−∞ :
-
area under the plasma concentration time curve from time zero to infinity
- AUClast :
-
area under the plasma concentration time curve from the last measurable concentration to infinity
- C max :
-
maximum plasma concentration
- t max :
-
time to reach maximum plasma concentrations
- t 1/2 :
-
plasma elimination half-life
- Cl:
-
confidence interval
- T:
-
test preparation
- R:
-
reference preparation
- ANOVA:
-
analysis of variance
References
Breithaupt H, Tittel M (1982) Kinetics of allopurinol after single intravenous and oral doses. Noninteraction with benzbromarone and hydrocblorothiazide. Eur J Clin Pharmacol 22:77–84
Bührens K, Berndt P, Hilgenstock CM, Baumann W, Janzen D (1991) Zum Nachweis der Biodquivalenz von zwei Allopurinol-Präparaten. Arzneimittelforschung 41:250–253
Chen ML, Jackson AJ (1991) The role of metabolites in bioequivalency assessment. 1. Linear pharmacokinetics without first-pass effect. Pharm Res 8:25–32
Currie WJC, Turmer P, Young JH (1978) Evaluation of once a day allopurinol administration in man. Br J Clin Pharmacol 5:90–91
de Vries JX, Voss A, Kutschker C, Reiter S (1993) The simultaneous determination of allopurinol and oxipurinol in human plasma and urine by high-performance liquid chromatography. Arzneimittelforschung (in press)
Drayer DE (1982) Pharmacologically active metabolites of drugs and other foreign compounds. Clinical, pharmacological, therapeutic and toxicological considerations. Drugs 24:519–542
Elion GB (1974) Drugs in the treatment of hyperuricemia. Adv Nephrol 3:51–57
Hande KR, Noone RM, Stone WJ (1984) Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med 76:47–56
Heinzel G, Woloszczak R, Thomann P (1992) TOPFIT, Version 2.0. Pharmacokinetic and pharmacodynamic data analysis system. Fischer, Stuttgart(in press)
Jaeger H, Russmann D, Rasper J, Blome J (1982) Vergleichende Prüfung der Bioverfügbarkeit und des pharmakodynamischen Effektes von für Allopurinolzubereitungen. Arzneimittelforschung 32:438–443
Marcus M, Tse FLS, Kleinberg SI (1982) The bioavailability of two commercial preparations of allopurinol tablets. Int J Clin Pharmacol Ther Toxicol 20:302–305
Matzkies F, Berg G (1974) Zur Wirkung einer täglichen Einzeldosis von 300 mg Allopurinol auf die Serumharnsäure und Uratausscheidung bei Gichtpatienten. Dtsch Med Wochenschr 99:2464–2465
Murrell GAC, Rapeport WG (1986) Clinical pharmacokinetics of allopurinol. Clin Pharmacokinet 11:343–353
Nightingale SL, Morrison JC (1987) Generic drugs and the prescribing physician. JAMA 258:1200–1204
Rodnan GP, Robin JA, Tolchin SF, Elion GB (1975) Allopurinol and gouty hyperuricemia. Efficacy of a single daily dose. JAMA 231:1143–1147
Stemijans VW, Hauschke D (1990) Update on the statistical analysis of bioequivalence studies. Int J Clin Pharmacol Ther Toxicol 28:105–110
Walter-Sack I, Gröbner W, Zöllner N (1979) Verlauf der Oxipurinol-Spiegel im Plasma nach akuter und chronischer Gabe von Allopurinol in verschiedenen galenischen Zubereitungen. Arzneimittelforschung 29:839–842
Zöllnert N, Griebsch A (1974) über eine Vereinfachung der Allopurmolbehandlung. München Med Wochenschr 116:1691–1692
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. Dr. N. Zöllner on the occasion of his 70th birthday
Rights and permissions
About this article
Cite this article
Walter-Sack, I., de Vries, J.X., Kreinerl, C. et al. Bioequivalence of allopurinol preparations: to be assessed by the parent drug or the active metabolite?. Clin Investig 71, 240–246 (1993). https://doi.org/10.1007/BF00180109
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00180109