Abstract
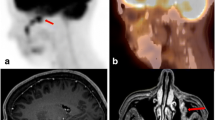
A positron emission tomography study using [18F]-fluorodeoxyglucose was undertaken to identify and quantitate whether diaschisis occurred in cerebral cortex, basal ganglia, or thalamus, as well as in cerebellar cortex and dentate nuclei in patients with malignant glioma. The relationship between diaschisis in these cerebral structures and clinically significant hemiparesis in patients was analysed. A 30% decrease in the regional metabolic rate for glucose in the cerebellar hemisphere contralateral to the tumor, and ipsilateral to the motor deficit, was identified and was statistically significant (p > 0.001). Decreased metabolism in the cerebellar hemisphere contralateral to the tumor was not seen in patients without hemiparesis. Parietal lobes affected by tumor had a larger decrease in metabolism than did frontal lobes with tumor (p > 0.01). The overall metabolism of the unaffected cerebellar hemisphere, relative to the peak metabolic activity of the brain, was not depressed in patients with tumor. In addition, the activity of subcortical nuclei was relatively unaffected by adjacent tumor or motor deficit.
Similar content being viewed by others
References
Feeney DM, Barron JC: Diaschisis. Stroke 17: 817–830, 1986
Lagréze HL, Levine RL, Pedula KL, Nickles RJ, Sunderland JS, Rowe BR: Contralateral flow reduction in unilateral stroke: Evidence for transhemispheric diaschisis. Stroke 18: 882–886, 1987
Kushner M, Alavi A, Reivich M, Dann R, Burke A, Robinson G: Contralateral cerebellar hypometabolism following cerebral insult: A positron emission tomographic study. Ann Neurol 15: 425–434, 1984
Wise RJ, Rhodes CG, Gibbs JM, Hatazawa J, Palmer T, Frackowiak RSJ, Jones T: Disturbance of oxidative metabolism of glucose in recent human cerebral infarcts. Ann Neurol 14: 627–637, 1983
Fukuyama H, Kameyama M, Harada K, Fujimoto N, Kobayashi A, Taki W, Ishikawa T, Handa H, Tanada S, Torizuka K: Thalamic tumours invading the brain stem produce crossed cerebellar diaschisis demonstrated by PET. J Neurol Neurosurg Psychiat 49: 524–528, 1986
Kempinskiary WH: Experimental study of diaschisis. Aruch Neurol Psychiat 79: 376–389, 1958
Plum F, Gjedde A, Samson FE: Neuro-anatomical functional mapping by the radioactive 2-DG method. Neurosci Res Progr Bull 14: (4) 455–518, 1976
Dauth G, Gilman S, Frey K, et al.: [14C]2-Deoxyglucose uptake in monkeys with hypotonic hemiplegia after precentral or postcentral lesions. Neurol 30: 407, 1980
Kennedy C, Miyaoka M, Suda S, et al.: Local metabolic responses in brain accompanying motor activity. Ann Neurol 8: 90, 1980
Baron JC, Bousser MG, Comar D, Duquesnoy N, Sastre J, Castaigne P: ‘Crossed cerebellar diaschisis’: A remote functional depression secondary to supratentorial infarction of man. JCBFM 1 (suppl): 500–501, 1981
Patronas NJ, Di Chiro G, Smith BH, DeLaPaz R, Brooks RA, Milam HL, Kornblith PL, Bairamian D, Mansi L: Depressed cerebellar glucose metabolism in supratentorial tumors. Brain Res 291: 93–101, 1984
Martin WRW, Raichle ME: Cerebellar blood flow and metabolism in cerebral hemisphere infarction. Ann Neurol 14: 168–176, 1983
Hawkins RA, Phelps ME, Huang SC: Effects of temporal sampling, glucose metabolic rates, and disruptions of the blood-brain barrier on the FDG model with and without a vascular compartment: Studies in human brain tumors with PET. JCBFM 6: 170–183, 1986
Brooks DJ, Beaney RP, Lammertsma AA, Herold S, Turton DR, Luthra SK, Frackowiak RSJ, Thomas DGT, Marshall J, Jones T: Glucose transport across the blood-brain barrier in normal human subjects and patients with cerebral tumors studied using [11C]3–0-methyl-D-glucose and positron emission tomography. JCBFM 6: 230–239, 1986
Leenders KL, Beaney RP, Brooks DJ, Lammertsma AA, Heather JD, McKenzie CG: Dexamethasone treatment of brain tumor patients: Effects on regional cerebral blood flow, blood volume, and oxygen utilization. Neurol 35: 1610–1616, 1985
Jarden JO, Dhawan V, Poltorak A, Posner JB, Rottenberg DA: Positron emission tomography measurement of blood-to-brain and blood-to-tumor transport of 82Rb; The effect of dexamethasone and whole-brain radiation therapy. Ann Neurol 18: 636–646, 1985
Tyler JL, Diksic M, Villemure J-G, Evan AC, Meyer E, Yamamoto YL, Feindel W: Metabolic and hemodynamic evaluation of gliomas using positron emission tomography. J Nucl Med 28: 1123–1133, 1987
Di Chiro G, Oldfield E, Wright DC, De Michele D, Katz DA, Patronas NJ, Doppman JL, Larson SM, Ito M, Kufta CV: Cerebral necrosis after radiotherapy and/or intraarterial chemotherapy for brain tumors: PET and neuropathologic studies. AJR 150: 189–197, 1988
Stroink AR, Hoffman HJ, Hendrick EB, Humphreys RP: Diagnosis and management of pediatric brain-stem gliomas. J Neurosurg 65: 745–750, 1986
Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH: The use of nitrogen mustards in the palliative treatment of carcinoma: With particular reference to bronchogenic carcinoma. Cancer 1: 634–656, 1948
Di Chiro G, DeLaPaz RL, Brooks RA, Sokoloff L, Kornblith PL, Smith BH, Patronas NJ, Kufta CV, Kessler RM, Johnston GS, Manning RG, Wolf AP: Glucose utilization of cerebral gliomas measured by [18F] fluorode-oxyglucose and positron emission tomography. Neurol 32: 1323–1329, 1982
Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE: Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: Validation of method. Ann Neurol 6: 371–388, 1979
Nelson T, Lucignani G, Goochee J, Crane AM, Sokoloff L: Invalidity of criticisms of the deoxyglucose method based on alleged glucose-6-phosphatase activity in brain. J Neurochem 46: 905–919, 1986
Scheffler WC: Statistics for health professionals. Addison-Wesley Publishing Company, Reading, Massachusetts. pp 151–177, 1984
Fox PT, Raichle ME, Mintun MA, Dence C: Nonoxidative glucose consumption during focal physiologic neural activity. Science 241: 462–464, 1988
Lou HC, Edvinsson L, MacKenzie ET: The concept of coupling blood flow to brain function: revision required? Ann Neurol 22: 289–297, 1987
DeLong MR, Alexander GE: Organization of basal ganglia. In: Asbury AK, McKhann Gm, McDonald WI (eds) Diseases of the Nervous System, WB Saunders Company, Philadelphia, Pennsylvania. pp 379–393, 1986
Thach WT: Cortical control of movement. In: Asbury AK, McKhann GM, McDonald WI (eds) Diseases of the Nervous System, W.B. Saunders Company, Philadelphia, Pennsylvania, pp 370–378, 1986
Georgopoulos AP, DeLong MR, Crutcher MD: Relations between parameters of step-tracking movements and single cell discharge in the globus pallidus and subthalamic nucleus of the behaving monkey. J Neurosci 3: 1586–1598, 1983
DeLong MR: Activity of pallidal neurons during movement. J Neurophysiol 34: 414–427, 1971
Kitai ST, Deniau JM: Cortical inputs to the subthalamus: Intracellular analysis. Brain Res 214: 411–415, 1981
Liles SL: Activity of neurons in putamen during active and passive movements of wrist. J Neurophysiol 53: 217–236, 1985
Crutcher MD, DeLong MR: Single cell studies of the primate putamen. II. Relations to direction of movement and pattern of muscular activity. Exp Brain Res 53: 244–258, 1984b
Chevalier G, Vacher S, Deniau JM, Desban M: Disinhibition as a basic process in the expression of striatal functions. I. The striato-nigral influence on tecto-spinal/tecto-diencephalic neurons. Brain Res 334: 215–226, 1985
Fonnum F, Gottesfeld Z, Grofova I: Distribution of glutamate decarboxylase, choline acetyltransferase and aromatic amino acid decarboxylase in the basal ganglia of normal and operated rats. Evidence for striatopallidal, striatoentopeduncular and striatonigral GABAergic fibres. Brain Res 143: 125–138, 1978
Deniau JM, Chevalier G: Disinhibition as a basic process in the expression of striatal functions. II. The striato-nigral influence on thalamocortical cells of the ventromedial thalamic nucleus. Brain Res 334: 227–233, 1985
MacLeod NK, James TA, Kilpatrick IC, Starr MS: Evidence for a GABAergic nigrothalamic pathway in the rat. II. Electrophysiological studies. Exp Brain Res 40: 55–61, 1980
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rozental, J.M., Levine, R.L., Nickles, R.J. et al. Cerebral diaschisis in patients with malignant glioma. J Neuro-Oncol 8, 153–161 (1990). https://doi.org/10.1007/BF00177839
Issue Date:
DOI: https://doi.org/10.1007/BF00177839