Summary
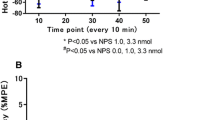
The study was carried out to provide further evidence that the two pyrazolone derivatives, metamizol and aminophenazone, produce central antinociceptive effects by stimulating inhibition descending from the periaqueductal grey (PAG) to the spinal cord. Experiments were carried out on rats in which the tail-flick response to radiant heat, nociceptive activity in ascending axons of the spinal cord, and activity of neurones in the PAG and the substantia nigra were studied. Microinjection of procaine (10 μg) into the PAG reduced the tail-flick latency and abolished the increase in latency caused by i.p. injection of metamizol (40 mg/kg) and aminophenazone (150 mg/kg); it did not significantly reduce the antinociceptive effect of i.p. injection of morphine (2 mg/kg). Threshold doses of morphine (1 and 2 μg) administered by intrathecal (i.t.) injection potentiated the effect of threshold doses of metamizol injected i.p. (10 mg/ kg) or into the PAG (10 μg) in the tail-flick test. Morphine (2 μg) injected i.t. potentiated the effect of i.v. injection of metamizol (80 mg/kg) on nociceptive activity in ascending axons by eliminating the stimulant effect of metamizol on about one third of the axons. Threshold doses of morphine injected i.t. failed to potentiate the antinociceptive effect of aminophenazone (50 mg/kg) injected i.p. in the tail-flick test. The results support the view that metamizol and aminophenazone activate pathways descending from the PAG and exerting an inhibitory effect on nociceptive impulse transmission at the spinal level.
Similar content being viewed by others
References
Balagura S, Ralph T (1973) The analgesic effect of electrical stimulation of the diencephalon and mesencephalon. Brain Res 60:369–379
Barasi S (1979) Responses of substantia nigra neurones to noxious stimulation. Brain Res 171:121–130
Brune K, Rainsford KD, Wagner K, Peskar BA (1981) Inhibition by anti-inflammatory drugs of prostaglandin production in cultured macrophages. Naunyn-Schmiedeberg's Arch Pharmacol 315:269–276
Carlsson K-H, Helmreich J, Jurna I (1986a) Activation of inhibition from the periaqueductal grey matter mediates central analgesic effect of metamizol (dipyrone). Pain (in press)
Carlsson K-H, Helmreich J, Jurna I (1986b) Comparison of central antinociceptive and analgesic effects of the pyrazolone derivatives, metamizol (dipyrone) and aminophenazone (“Pyra-midon”). Schmerz-Pain-Douleur 7:93–100
Doi T, Jurna I (1982) Analgesic effect of intrathecal morphine demonstrated in ascending nociceptive activity in the rat spinal cord and ineffectiveness of caerulein and cholecystokinin octapeptide. Brain Res 234:399–407
Driesen W, Hahn F, Rummel W (1950) Über den Einfluß von Cardiazol, Coramin und Pyramidon auf das Elektroencephalogramm (E.E.G.) und das Myelogramm von Katzen und Kaninchen. Dtsch Z Nervenheilk 164:395–406
Fifková E, Maršala J (1967) Stereotaxic atlases for the cat, rabbit and rat. In: Bureš J, Petrán M, Zachar J (eds) Electrophysiological methods in biological research. Academic Press, New York, pp 653–731
Gebhart GF (1982) Opiate and opioid peptide effects on brain stem neurons: relevance to nociception and antinociceptive pathways. Pain 12:93–140
Gebhart GF, Sandkühler J, Thalhammer JG, Zimmermann M (1983) Inhibition of spinal nociceptive information by stimulation in midbrain of the cat is blocked by lidocaine microinjected in nucleus raphe magnus and medullary reticular formation. J Neurophysiol 50:1446–1459
Jurna I (1963) Die Wirkung von 1-Phenyl-2,3-dimethyl-4-dimethylaminopyrazolon (Pyramidon) und 1-Phenyl-2,3-dimethyl-4isopropylaminopyrazolon (Isopyrin) auf spinale Reflexe. Naunyn-Schmiedeberg's Arch Pharmacol 245:305–320
Jurna I (1972) Dämpfung repetitiver Aktivierungsvorgänge an der spinalen Motorik durch Morphin. In: Janzen R, Keidel WD, Herz A, Steichele C, Payne JP, Burt RA (eds) Schmerz (Pain). Thieme, Stuttgart, pp 267–269
Jurna I (1980) Effect of stimulation in the periaqueductal grey matter on activity in ascending axons of the rat spinal cord: selective inhibition of activity evoked by afferent Aδ and C fibre stimulation and failure of naloxone to reduce inhibition. Brain Res 196:33–42
Jurna I, Heinz G (1979) Differential effects of morphine and opioid analgesics on A and C fibre-evoked activity in ascending axons of the rat spinal cord. Brain Res 171:573–576
Jurna I, Heinz G, Blinn G, Nell T (1978) The effect of substantia nigra stimulation and morphine on α-motoneurones and the tail-flick response. Naunyn-Schmiedeberg's Arch Pharmacol 51:239–250
Jurna I, Zetler G (1981) Antinociceptive effect of centrally administered caerulein and cholecystokinin octapeptide (CCK8). Eur J Pharmacol 73:323–331
Jurna I, Zetler G (1985) Effects on ascending nociceptive activity in the rat spinal cord produced by cholecystokinin octapeptide, ceruletide, and morphine injected into the periaqueductal gray matter. Ann NY Acad Sci 448:609–612
Lewis VA, Gebhart GF (1977) Evaluation of the periaqueductal central gray (PAG) as a morphine-specific locus of action and examination of morphine-induced and stimulation produced analgesia at coincident PAG loci. Brain Res 124:283–303
Mayer DJ, Wolfle H, Akil H, Carder B, Liebeskind JC (1971) Analgesia from electrical stimulation in the brain stem of the rat. Science 174:1351–1354
Pay S, Barasi S (1982) A study of the connections of nociceptive substantia nigra neurones. Pain 12:75–89
Reynolds DV (1969) Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science 164:444–445
Starkenstein E, Hendrych F, Escobar-Bordoy J (1934) Zur experimentellen Analyse der Pyramidonwirkung. Naunyn-Schmiedeberg's Arch Pharmacol 176:486–493
Weithmann KU, Alpermann H-G (1985) Biochemical and pharmacological effects of dipyrone and its metabolites in model systems related to arachidonic acid cascade. Arzneimittelforsch 35:947–952
Wilcox GL, Carlsson K-H, Jochim A, Jurna I (1986) Mutual potentiation of antinociceptive effects of morphine and clonidine on motor and sensory responses in rat spinal cord. Brain Res (in press)
Yaksh TL, Rudy TA (1976a) Chronic catheterization of the spinal subarachnoid space. Physiol Behav 17:1031–1036
Yaksh TL, Rudy TA (1976b) Analgesia mediated by a direct spinal action of narcotics. Science 192:1357–1358
Yaksh TL, Rudy TA (1978) Narcotic analgesics: CNS sites and mechanisms of action as revealed by intracerebral injection techniques. Pain 4:299–359
Yeung JC, Rudy TA (1980) Multiplicative interaction between narcotic agonisms expressed at spinal and supraspinal sites of antinociceptive action as revealed by concurrent intrathecal and intracerebroventricular injections of morphine. J Pharmacol Exp Ther 215:633–642
Zieglgänsberger E (1985) Opioid actions on mammalian spinal neurones. Int Rev Neurobiol 25:243–275
Author information
Authors and Affiliations
Additional information
Supported by the Schwerpunkt “Nociception and Schmerz” of the Deutsche Forschungsgemeinschaft
Send offprint requests to I. Jurna at the above address
Rights and permissions
About this article
Cite this article
Carlsson, KH., Jurna, I. The role of descending inhibition in the antinociceptive effects of the pyrazolone derivatives, metamizol (dipyrone) and aminophenazone (“Pyramidon”). Naunyn-Schmiedeberg's Arch Pharmacol 335, 154–159 (1987). https://doi.org/10.1007/BF00177717
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00177717