Abstract
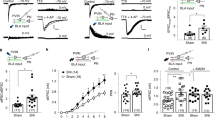
The ability to modulate pain perception is as critical to survival as pain itself. The most known pain modulation pathway is the PAG–RVM (periaqueductal gray–rostral ventromedial medulla) descending system. In this study, we hypothesized that it is functionally linked to the ascending nociceptive control, which is a form of pain-induced analgesia dependent on mesolimbic mechanisms. To test this hypothesis, we used a pharmacological approach, in which the antinociception induced by noxious stimulation (forepaw injection of capsaicin) was detected in a standard rat model of inflammatory pain (hindpaw injection of carrageenan). This antinociception was blocked by interventions known to block the ascending nociceptive control-mediated analgesia: the blockade of μ-opioid (Cys2,Tyr3,Orn5,Pen7amide (CTOP) 0.5 μg) or of dopamine (SCH23390 1.8 μg and raclopride 5 μg) receptors within the NAc (nucleus accumbens) and that of cholinergic nicotinic receptors (mecamylamine 0.6 μg) within the RVM. The antinociception was also blocked by standard interventions known to block mechanisms of descending inhibition within either the PAG or the RVM: local acute neuronal blockade (lidocaine 2%), blockade of μ-opioid receptors (CTOP 0.5 μg), or activation of GABAA receptors (muscimol 10 ng). Consistently, interventions that are known to block spinal mechanisms of descending inhibition also blocked antinociception: lesion of dorsolateral funiculus and the spinal blockade of serotonergic (WAY100135 46 μg or tropisetron 10 μg) or adrenergic (idazoxan, 50 μg) receptors. Neuronal activity indirectly estimated by c-Fos expression within the NAc, PAG, and RVM supports behavioral observations. Therefore, this study provides functional data indicating that noxious stimulation triggers an ascending–descending pain modulation pathway linking the mesolimbic system to the PAG–RVM descending system.






Similar content being viewed by others
References
Porreca F, Navratilova E (2017) Reward, motivation, and emotion of pain and its relief. Pain 158(Suppl 1):S43–S49. https://doi.org/10.1097/j.pain.0000000000000798
Ossipov MH, Dussor GO, Porreca F (2010) Central modulation of pain. J Clin Invest 120(11):3779–3787. https://doi.org/10.1172/JCI43766
Gaskin DJ, Richard P (2012) The economic costs of pain in the United States. J Pain : Off J Am Pain Soc 13(8):715–724. https://doi.org/10.1016/j.jpain.2012.03.009
Kennedy J, Roll JM, Schraudner T, Murphy S, McPherson S (2014) Prevalence of persistent pain in the U.S. adult population: new data from the 2010 national health interview survey. J Pain : Off J Am Pain Soc 15(10):979–984. https://doi.org/10.1016/j.jpain.2014.05.009
Melzack R, Wall PD (1965) Pain mechanisms: a new theory. Science 150(3699):971–979
Fields H (2004) State-dependent opioid control of pain. Nat Rev Neurosci 5(7):565–575. https://doi.org/10.1038/nrn1431
Millan MJ (2002) Descending control of pain. Prog Neurobiol 66(6):355–474
Gear RW, Aley KO, Levine JD (1999) Pain-induced analgesia mediated by mesolimbic reward circuits. J Neurosci 19(16):7175–7181
Gear RW, Levine JD (1995) Antinociception produced by an ascending spino-supraspinal pathway. J Neurosci 15(4):3154–3161
Gear RW, Levine JD (2009) Rostral ventral medulla cholinergic mechanism in pain-induced analgesia. Neurosci Lett 464(3):170–172. https://doi.org/10.1016/j.neulet.2009.08.036
Baliki MN, Apkarian AV (2015) Nociception, pain, negative moods, and behavior selection. Neuron 87(3):474–491. https://doi.org/10.1016/j.neuron.2015.06.005
Bonet IJ, Fischer L, Parada CA, Tambeli CH (2013) The role of transient receptor potential A 1 (TRPA1) in the development and maintenance of carrageenan-induced hyperalgesia. Neuropharmacology 65:206–212. https://doi.org/10.1016/j.neuropharm.2012.09.020
Ozdemir E, Gursoy S, Bagcivan I (2012) The effects of serotonin/norepinephrine reuptake inhibitors and serotonin receptor agonist on morphine analgesia and tolerance in rats. J Physiol Sci : JPS 62(4):317–323. https://doi.org/10.1007/s12576-012-0207-x
Schmidt BL, Tambeli CH, Levine JD, Gear RW (2002) Mu/delta cooperativity and opposing kappa-opioid effects in nucleus accumbens-mediated antinociception in the rat. Eur J Neurosci 15(5):861–868
Tobaldini G, Aisengart B, Lima MM, Tambeli CH, Fischer L (2014) Ascending nociceptive control contributes to the antinociceptive effect of acupuncture in a rat model of acute pain. J Pain : Off J Am Pain Soc 15(4):422–434. https://doi.org/10.1016/j.jpain.2013.12.008
Sardi NF, Tobaldini G, Morais RN, Fischer L (2017) Nucleus Accumbens mediates the pronociceptive effect of sleep deprivation: the role of adenosine A2A and dopamine D2 receptors. Pain 159:75–84. https://doi.org/10.1097/j.pain.0000000000001066
Holden JE, Farah EN, Jeong Y (2005) Stimulation of the lateral hypothalamus produces antinociception mediated by 5-HT1A, 5-HT1B and 5-HT3 receptors in the rat spinal cord dorsal horn. Neuroscience 135(4):1255–1268. https://doi.org/10.1016/j.neuroscience.2005.07.023
Bardin L, Jourdan D, Alloui A, Lavarenne J, Eschalier A (1997) Differential influence of two serotonin 5-HT3 receptor antagonists on spinal serotonin-induced analgesia in rats. Brain Res 765(2):267–272
Silva JR, Silva ML, Prado WA (2011) Analgesia induced by 2- or 100-Hz electroacupuncture in the rat tail-flick test depends on the activation of different descending pain inhibitory mechanisms. J Pain : Off J Am Pain Soc 12(1):51–60. https://doi.org/10.1016/j.jpain.2010.04.008
Viisanen H, Ansah OB, Pertovaara A (2012) The role of the dopamine D2 receptor in descending control of pain induced by motor cortex stimulation in the neuropathic rat. Brain Res Bull 89(3–4):133–143. https://doi.org/10.1016/j.brainresbull.2012.08.002
Schmidt BL, Tambeli CH, Barletta J, Luo L, Green P, Levine JD, Gear RW (2002) Altered nucleus accumbens circuitry mediates pain-induced antinociception in morphine-tolerant rats. J Neurosci 22(15):6773–6780
Tomim DH, Pontarolla FM, Bertolini JF, Arase M, Tobaldini G, Lima MM, Fischer L (2016) The pronociceptive effect of paradoxical sleep deprivation in rats: evidence for a role of descending pain modulation mechanisms. Mol Neurobiol 53(3):1706–1717. https://doi.org/10.1007/s12035-014-9059-0
Araldi D, Ferrari LF, Lotufo CM, Vieira AS, Athie MC, Figueiredo JG, Duarte DB, Tambeli CH et al (2013) Peripheral inflammatory hyperalgesia depends on the COX increase in the dorsal root ganglion. Proc Natl Acad Sci U S A 110(9):3603–3608. https://doi.org/10.1073/pnas.1220668110
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. 6th edn. Academic Press, New York
Dall'Acqua MC, Bonet IJ, Zampronio AR, Tambeli CH, Parada CA, Fischer L (2014) The contribution of transient receptor potential ankyrin 1 (TRPA1) to the in vivo nociceptive effects of prostaglandin E(2). Life Sci 105(1–2):7–13. https://doi.org/10.1016/j.lfs.2014.02.031
Randall LO, Selitto JJ (1957) A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther 111(4):409–419
Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Hansson P, Lautenbacher S et al (2010) Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain 14(4):339. https://doi.org/10.1016/j.ejpain.2010.02.004
Pud D, Granovsky Y, Yarnitsky D (2009) The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain 144(1–2):16–19. https://doi.org/10.1016/j.pain.2009.02.015
Tambeli CH, Levine JD, Gear RW (2009) Centralization of noxious stimulus-induced analgesia (NSIA) is related to activity at inhibitory synapses in the spinal cord. Pain 143(3):228–232. https://doi.org/10.1016/j.pain.2009.03.005
Le Bars D (2002) The whole body receptive field of dorsal horn multireceptive neurones. Brain Res Rev 40(1–3):29–44
Lane DA, Patel PA, Morgan MM (2005) Evidence for an intrinsic mechanism of antinociceptive tolerance within the ventrolateral periaqueductal gray of rats. Neuroscience 135(1):227–234. https://doi.org/10.1016/j.neuroscience.2005.06.014
Lau BK, Vaughan CW (2014) Descending modulation of pain: the GABA disinhibition hypothesis of analgesia. Curr Opin Neurobiol 29:159–164. https://doi.org/10.1016/j.conb.2014.07.010
Vaughan CW, Ingram SL, Connor MA, Christie MJ (1997) How opioids inhibit GABA-mediated neurotransmission. Nature 390(6660):611–614. https://doi.org/10.1038/37610
Yaksh TL, Yeung JC, Rudy TA (1976) Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res 114(1):83–103
Meng ID, Johansen JP, Harasawa I, Fields HL (2005) Kappa opioids inhibit physiologically identified medullary pain modulating neurons and reduce morphine antinociception. J Neurophysiol 93(3):1138–1144. https://doi.org/10.1152/jn.00320.2004
Budai D, Fields HL (1998) Endogenous opioid peptides acting at mu-opioid receptors in the dorsal horn contribute to midbrain modulation of spinal nociceptive neurons. J Neurophysiol 79(2):677–687
Kiefel JM, Rossi GC, Bodnar RJ (1993) Medullary mu and delta opioid receptors modulate mesencephalic morphine analgesia in rats. Brain Res 624(1–2):151–161
Gear RW, Levine JD (2011) Nucleus accumbens facilitates nociception. Exp Neurol 229(2):502–506. https://doi.org/10.1016/j.expneurol.2011.03.021
Han F, Zhang YF, Li YQ (2003) Fos expression in tyrosine hydroxylase-containing neurons in rat brainstem after visceral noxious stimulation: an immunohistochemical study. World J Gastroenterol 9(5):1045–1050
Keay KA, Bandler R (1993) Deep and superficial noxious stimulation increases Fos-like immunoreactivity in different regions of the midbrain periaqueductal grey of the rat. Neurosci Lett 154(1–2):23–26
Loyd DR, Morgan MM, Murphy AZ (2007) Morphine preferentially activates the periaqueductal gray-rostral ventromedial medullary pathway in the male rat: a potential mechanism for sex differences in antinociception. Neuroscience 147(2):456–468. https://doi.org/10.1016/j.neuroscience.2007.03.053
Berridge KC, Kringelbach ML (2015) Pleasure systems in the brain. Neuron 86(3):646–664. https://doi.org/10.1016/j.neuron.2015.02.018
Navratilova E, Atcherley CW, Porreca F (2015) Brain circuits encoding reward from pain relief. Trends Neurosci 38(11):741–750. https://doi.org/10.1016/j.tins.2015.09.003
De Felice M, Ossipov MH (2016) Cortical and subcortical modulation of pain. Pain Manag 6(2):111–120. https://doi.org/10.2217/pmt.15.63
Suckow SK, Deichsel EL, Ingram SL, Morgan MM, Aicher SA (2013) Columnar distribution of catecholaminergic neurons in the ventrolateral periaqueductal gray and their relationship to efferent pathways. Synapse 67(2):94–108. https://doi.org/10.1002/syn.21624
Kirouac GJ, Li S, Mabrouk G (2004) GABAergic projection from the ventral tegmental area and substantia nigra to the periaqueductal gray region and the dorsal raphe nucleus. J Comp Neurol 469(2):170–184. https://doi.org/10.1002/cne.11005
Ma QP, Han JS (1991) Neurochemical studies on the mesolimbic circuitry of antinociception. Brain Res 566(1–2):95–102
Ma QP, Shi YS, Han JS (1992) Further studies on interactions between periaqueductal gray, nucleus accumbens and habenula in antinociception. Brain Res 583(1–2):292–295
Yu LC, Han JS (1990) Habenula as a relay in the descending pathway from nucleus accumbens to periaqueductal grey subserving antinociception. Int J Neurosci 54(3–4):245–251
Sprenger C, Bingel U, Buchel C (2011) Treating pain with pain: supraspinal mechanisms of endogenous analgesia elicited by heterotopic noxious conditioning stimulation. Pain 152(2):428–439. https://doi.org/10.1016/j.pain.2010.11.018
Yelle MD, Oshiro Y, Kraft RA, Coghill RC (2009) Temporal filtering of nociceptive information by dynamic activation of endogenous pain modulatory systems. J Neurosci 29(33):10264–10271. https://doi.org/10.1523/JNEUROSCI.4648-08.2009
Derbyshire SW, Osborn J (2009) Offset analgesia is mediated by activation in the region of the periaqueductal grey and rostral ventromedial medulla. NeuroImage 47(3):1002–1006. https://doi.org/10.1016/j.neuroimage.2009.04.032
Harris JA (1996) Descending antinociceptive mechanisms in the brainstem: their role in the animal’s defensive system. J Physiol Paris 90(1):15–25
Tambeli CH, Fischer L, Monaliza SL, Menescal-de-Oliveira L, Parada CA (2012) The functional role of ascending nociceptive control in defensive behavior. Brain Res 1464:24–29. https://doi.org/10.1016/j.brainres.2012.05.010
Leite-Panissi CR, Coimbra NC, Menescal-de-Oliveira L (2003) The cholinergic stimulation of the central amygdala modifying the tonic immobility response and antinociception in guinea pigs depends on the ventrolateral periaqueductal gray. Brain Res Bull 60(1–2):167–178
Acknowledgments
This study was supported by the National Council for Scientific and Technological Development (CNPq, Brazil), Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil), and Funding Authority for Studies and Projects (FINEP, Brazil). G.T. and N.F.S. were recipient of PhD fellowships from CAPES.
Funding
The authors declare no source of funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Electronic Supplementary Material
Supplementary Table 1
Effect (mean ± S.E.M.) of experimental manipulations on locomotor activity in the open field test. None of the treatments significantly affected locomotion (p > 0.05, one-way ANOVA). N/A = not applicable; vehicle = 0.9% NaCl; NAc = nucleus accumbens; PAG = periaqueductal gray; RVM = rostral ventromedial medulla; DLF = dorsolateral funiculus. (XLSX 11 kb)
Supplementary Figure S1
Capsaicin-induced antinociception is similar to systemic morphine-induced antinociception. The forepaw injection of capsaicin or the systemic morphine administration significantly increased (indicated by the symbol “*”) mechanical nociceptive threshold in animals that have received carrageenan into the hindpaw (repeated-measures ANOVA and Student-Newman-Keuls post hoc test, p < 0.05). The groups 100 μg carrageenan s.c. hindpaw+0.9% NaCl s.c. forepaw and 100 μg carrageenan s.c. hindpaw +250 μg capsaicin s.c. forepaw were replotted from Fig. 2A. (PNG 66.8 kb)
Supplementary Figure S2
Control groups – by themselves the experimental manipulations did not affect carrageenan-induced hyperalgesia. (A) The hindpaw injection of carrageenan dose dependently decreased the mechanical nociceptive threshold. Carrageenan at 20 μg induced an intermediate response which facilitates the observation of any tendency of drugs to further decrease (or increase) mechanical nociceptive threshold (the symbol “#” indicates mechanical nociceptive threshold significantly different from the other groups; the symbol “+” indicates a significant decrease in mechanical nociceptive threshold compared with the saline group,). The administration of the drugs used in this study within the (B) NAc (nucleus accumbens), (C) PAG (periaqueductal gray), (D) RVM (rostral ventromedial medulla) or (E) spinal cord (including DLF lesion) did not affect carrageenan-induced hyperalgesia (repeated-measures ANOVA and Student-Newman-Keuls post hoc test, p < 0.05). (PNG 274 kb)
Supplementary Figure S3
Capsaicin-induced antinociception is similar to intra-PAG DAMGO-induced antinociception. The forepaw injection of capsaicin or the intra-PAG administration of DAMGO (a μ-opioid receptor agonist) significantly increased (indicated by the symbol “*”) mechanical nociceptive threshold in animals that have received saline into the hindpaw (repeated-measures ANOVA and Student-Newman-Keuls post hoc test, p < 0.05). The groups 0.9% NaCl s.c. hindpaw+0.9% NaCl s.c. forepaw and 0.9% NaCl s.c. hindpaw+250 μg capsaicin s.c. forepaw were replotted from Fig. 2A. (PNG 61.4 kb)
Supplementary Figure S4
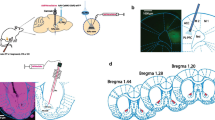
Microinjection sites. (A) Reconstruction adapted from the atlas of Paxinos and Watson showing microinjections within the NAc core, (B) ventrolateral PAG and (C) RVM. Some symbols overlap others and represent injection sites in two representative groups: “●” on-site injections (animals included in the study); “Δ” off-site injections (animals excluded from the study). Numbers represent distance caudal to bregma in millimeters. (PNG 70.1 kb)
Rights and permissions
About this article
Cite this article
Tobaldini, G., Sardi, N.F., Guilhen, V.A. et al. Pain Inhibits Pain: an Ascending-Descending Pain Modulation Pathway Linking Mesolimbic and Classical Descending Mechanisms. Mol Neurobiol 56, 1000–1013 (2019). https://doi.org/10.1007/s12035-018-1116-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1116-7