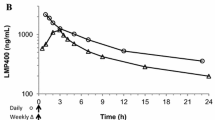
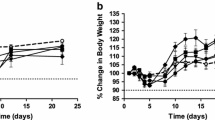
Abstract
Topotecan, irinotecan, and 9-aminocamptothecin (9-AC) are analogs of the plant alkaloid 20(S)-camptothecin (CMT), the prototypical DNA topoisomerase I interactive agent. These agents interact with the topoisomerase I-DNA complex and prevent resealing topoisomerase I-mediated DNA single-strand breaks. This eventual leads to double-strand DNA breaks and apoptosis or cell death. Topotecan, irinotecan, and 9-AC have shown significant activity in mice bearing pediatric solid tumor xenografts; the greatest antitumor responses were found with protracted continuous schedules. Preclinical data also suggest that maintenance of an exposure-duration threshold (EDT) may be required to achieve optimal cytotoxicity. Pediatric Phase I trials have evaluated the toxicity and safety of camptothecin analogs in children with relapsed solid tumors and relapsed acute leukemia. The primary dose-limiting toxicity (DLT) for the CMT analogs in children has been myelosuppression, except for mucositis observed with the 120-hr continuous topotecan infusion schedule. Pharmacodynamic relationships with these analogs have been reported between systemic exposure, and myelosuppression and mucositis. Although not a primary objective of the early Phase I studies, antitumor responses have been reported. In this review, the pharmacokinetics and pharmacodynamics of the CMT analogs studied in children are summarized, and future studies of these agents are discussed.
Similar content being viewed by others
References
Wall ME, Wani MC: Antineoplastic agents from plants. Annu Rev Pharmacol Toxicol 17:117–132, 1977.
Gupta M, Fujimori A, Pommier Y: Eukaryotic DNA topoisomerase I. Biochimica et Biophysica Acta 1262:1–14, 1995.
Giovanella BC, Stehlin JS, Wall ME, Wani MC, Nicholas AW, Liu LF, Silber R, Potmesil M: DNA topoisomerase I-targeted chemotherapy of human colon cancer in xenografts. Science 246:1046–1048, 1989.
Husain I, Mohler JL, Seigler HF, Besterman JM: Elevation of topoisomerase I messenger RNA, protein, and catalytic activity in human tumors: demonstration of tumor-type specificity and implications for cancer chemotherapy. Cancer Research 54:539–546, 1994.
Baker SD, Wadkins RM, Stewart CF, Beck WT, Danks MK: Cell cycle analysis of amount and distribution of nuclear DNA topoisomerase I as determined by fluorescence digital imaging microscopy. Cytometry 19:134–145, 1995.
Potmesil M: Camptothecins: From bench research to hospital wards. Cancer Research 54:1431–1439, 1994.
Smith PJ, Soues S: Multilevel therapeutic targeting by topoisomerase inhibitors. British Journal of Cancer 23:S47-S51, 1994.
Chen AY, Liu LF: DNA topoisomerases: essential enzymes and lethal targets. Annu Rev Pharmacol Toxicol 34:191–218, 1994.
Wall ME, Wani MC: Camptothecin and taxol: discovery to clinic — Thirteenth Bruce F. Cain Memorial Award Lecture. Cancer Research 55:753–760, 1995.
Abigerges D, Chabot GG, Armand JP, Herait P, Gouyette A, Gandia D: Phase I and pharmacologic studies of the camptothecin analog irinotecan administered every 3 weeks in cancer patients. J Clin Oncol 13:210–221, 1995.
Rivory LP, Robert J: Identification and kinetics of a beta-glucuronide metabolite of SN-38 in human plasma after administration of the camptothecin derivative irinotecan. Cancer Chemother Pharmacol 36:176–179, 1995.
Gupta E, Lestingi TM, Mick R, Ramirez J, Yokes EE, Ratain MJ: Metabolic fate of irinotecan in humans: correlation of glucuronidation with diarrhea. Cancer Res 54:3723–3725, 1994.
Tanizawa A, Fujimori A, Fujimori Y, Pommier Y: Comparison of topoisomerase I inhibition, DNA damage, and cytotoxicity of camptothecin derivatives presently in clinical trials. Journal of the National Cancer Institute 86:836–842, 1994.
Pommier Y, Leteurtre F, Fesen MR, Fujimori A, Bertrand R, Solary E, Kohlhagen G, Kohn KW: Cellular determinants of sensitivity and resistance to DNA topoisomerase inhibitors. Cancer Investigation 12:530–542, 1994.
Beijnen JH, Smith BR, Keijer WJ, Van Gijn R, Huinink WW, Vlasveld LT, Rodenhuis S, Underberg WJM: High-performance liquid chromatographic analysis of the new antitumour drug SK&F 104864-A (NSC 609699) in plasma. J Pharm Biomed Anal 8:789–794, 1990.
Burris HA, III, Hanauske AR, Johnson RK, Marshall MH, Kuhn JG, Hilsenbeck SG, Von Hoff DD: Activity of topotecan, a new topoisomerase I inhibitor, against human tumor colony-forming units in vitro. J Natl Cancer Inst 84:1816–1820, 1992.
Houghton PJ, Cheshire PJ, Lutz L, Stewart CF, Luo X, McGovern JP, Houghton JA: Efficacy of orally administered irinotecan against human colon tumor xenograft models. Clinical Cancer Research 1996 (in review).
Houghton PJ, Cheshire PJ, Myers L, Stewart CF, Synold TW, Houghton JA: Evaluation of 9-dimethylaminomethyl-10-hydroxycamptothecin against xenografts derived from adult and childhood solid tumors. Cancer Chemother Pharmacol 31:229–239, 1992.
Houghton PJ, Cheshire PJ, Hallman JD, Lutz L, Friedman HS, Danks MK, Houghton JA: Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice bearing xenografts of human tumors. Cancer Chemotherapy and Pharmacology 36:393–403, 1995.
Friedman HS, Houghton PJ, Schold SC, Keir S, Bigner DD: Activity of 9-dimethylaminomethyl-10-hydroxy-camptothecin against pediatric and adult central nervous system tumor xenografts. Cancer Chemotherapy and Pharmacology 34:171–174, 1994.
Komuro H, Li P, Tsuchida Y, Yokomri K, Nakajima K, Aoyama T, Kaneko M, Kaneda N: Effects of CPT-11 (a unique DNA topoisomerase inhibitor) on a highly malignant xeno-transplated neuroblastoma. Med Fed Onc 23:487–492, 1994.
Thompson J, Cheshire PJ, Lutz L, Luo X, Li Y, Houghton JA, Houghton PJ: Efficacy of systemic or oral administration of irinotecan against neuroblastoma xenografts. Clinical Cancer Research 1996 (in review).
Houghton PJ, Cheshire PJ, Hallman JC, Bissery MC, Mathieu-Boue A, Houghton JA: Therapeutic efficacy of the topoisomerase I inhibitor 7-ethyl-10-(4-[1-piperi-dino]-1-piperidino)-carbonyloxy-camptothecin against human tumor xenografts: lack of cross-resistance in vivo in tumors with acquired resistance to the topoisomerase I inhibitor 9-dimethylaminomethyl-10-hydroxycamptothecin. Cancer Research 53:2823–2829, 1993.
Slichenmyer WJ, Rowinsky EK, Donehower RC, Kaufmann SH: The current status of camptothecin analogues as antitumor agents. J Natl Cancer Inst 85:271–291, 1993.
Burris HA, Fields SM: Topoisomerase I inhibitors: an overview of the camptothecin analogs. Hematology/Oncology Clinics of North America 8:333–355, 1994.
Blaney SM, Balis FM, Cole DE, Craig C, Reid JM, Ames MM, Krailo M, Reaman G, Hammond D, Poplack DG: Pediatric phase I trial and pharmacokinetic study of topotecan administered as a 24-hour continuous infusion. Cancer Res 53:1032–1036, 1993.
Pratt CB, Stewart C, Santana VM, Bowman L, Furman W, Ochs J, Marina N, Kuttesch JF, Heideman R, Sandlund JT, et al.: Phase I study of topotecan for pediatric patients with malignant solid tumors. J Clin Oncol 12:539–543, 1994.
Stewart CF, Baker SD, Heideman RL, Jones D, Crom WR, Pratt CB: Clinical pharmacodynamics of continuous infusion topotecan in children: systemic exposure predicts hematologic toxicity. J Clin Oncol 12:1946–1954, 1994.
Uckun FM, Stewart CF, Reaman G, Chelstrom LM, Jin J, Chandan-Langlie M, Waddick KG, White J, Evans WE: In vitro and in vivo activity of topotecan against human B-lineage acute lymphoblastic leukemia cells. Blood 85:2817–2828, 1995.
Furman WL, Baker SD, Pratt CB, Rivera G, Evans WE, Stewart CF: Escalating systemic exposure to topotecan following a 120-hr continuous infusion in children with relapsed acute leukemia. Journal of Clinical Oncology 14:1504–1511, 1996.
Evans WE, Rodman JH, Relling MV, Crom WR, Rivera GK, Pratt CB: Concept of maximum tolerated systemic exposure and its application to Phase I–II studies of anticancer drugs. Med Fed Onc 19:153–159, 1991.
Kantarjian HM, Beran M, Ellis A, Zwelling L, O'Brien S, Cazenave L, Koller C, Rios MB, Plunkett W, Keating MJ, et al.: Phase I study of Topotecan, a new topoisomerase I inhibitor, in patients with refractory or relapsed acute leukemia. Blood 81:1146–1151, 1993.
Rowinsky EK, Adjei A, Donehower RC, Gore SD, Jones RJ, Burke PJ, Cheng YC, Grochow LB, Kaufmann SH: Phase I and pharmacodynamic study of the topoisomerase I-inhibitor topotecan in patients with refractory acute leukemia. J Clin Oncol 12:2193–2203, 1994.
Van Warmerdam LJ, Verweij J, Schellens JH, Rosing H, Davies BE, de Boer-Dennert M, Maes RA, Beijnen JH: Pharmacokinetics and pharmacodynamics of topotecan administered daily for 5 days every 3 weeks. Cancer Chemother Pharmacol 35:237–245, 1995.
Tubergen D, Pratt C, Stewart C, Vietti T: Phase I study of topotecan in children with refractory solid tumors: a Pediatric Oncology Group study. Proc Annu Meet Am Soc Clin Oncol 13:A463, 1994.
Cheng MF, Chatterjee S, Berger NA: Schedule-dependent cytotoxicity of topotecan alone and in combination chemotherapy regimens. Oncol Res 6:269–279, 1994.
Heideman R, Kuttesch J, Stewart C, Meyer W, Bowman L, Furman W, Gajjar A, Avery L, Pratt C: A phase I trial of a fixed systemic exposure (AUC) or carboplatin (CARBO) with continuous infusion (CI) topotecan (TOPO) in pediatric solid tumors. Proc Am Soc Clin Oncol 14:447, 1995.
Saylors RL, Stewart CF, Wall DA, Bell B, Shuster J, Pratt CB, Vietti TJ: Phase I trial of topotecan plus cyclophosphamide in children with refractory or recurrent solid tumors. A Pediatric Oncology Group (POG) study (POG 9375). American Journal of Pediatric Hematology/Oncology 1995 (abstract).
Bowman LC, Stewart CF, Zamboni WC, Crom WR, Luo X, Heideman R, Houghton PJ, Meyer WH, Pratt CB: Toxicity and pharmacodynamics of oral topotecan (POTOPO) in pediatric patients with relapsed solid tumors. Proceeding of American Society of Clinical Oncology 15: 1996.
Zamboni WC, Crom WR, Bowman LC, Pratt CB, Houghton PJ, Stewart CF: Interpatient variability in oral (PO) absorption of topotecan (TPT) in children with relapsed solid tumors. Clin Pharmacol Ther 59:198, 1996.
Zamboni WC, Heideman RL, Meyer WH, Gajjar AJ, Crom WR, Stewart CF: Pharmacokinetics (PK) of topotecan in pediatric patients with normal and altered renal function. Proceedings of American Society of Clinical Oncology 15: 1996 (abstract).
Slichenmyer W, Chen TL, Donehower R, Sartorius S, Rowinsky E, Shifflett C, Bowling K, Grochow L: Clinical pharmacology of topotecan in cancer patients with renal or hepatic dysfunction. Proc Annu Meet Am Soc Clin Oncol 13:A363, 1994.
Blaney SM, Cole DE, Balis FM, Godwin K, Poplack DG: Plasma and cerebrospinal fluid pharmacokinetic study of topotecan in nonhuman primates. Cancer Res 53:725–727, 1993.
Blaney SM, Cole DE, Godwin K, Sung C, Poplack DG, Balis FM: Intrathecal administration of topotecan in nonhuman primates. Cancer Chemother Pharmacol 36:121–124, 1995.
Baker SD, Heideman RL, Crom WR, Kuttesch JF, Gajjar A, Stewart CF: Cerebrospinal pharmacokinetics and pentration of continuous infusion topotecan in children with central nervous system tumors. Cancer Chemotherapy and Pharmacology 37:195–202, 1996.
Author information
Authors and Affiliations
Additional information
Address for offprints: Clinton F. Stewart, Department of Pharmaceutical Sciences, St. Jude Children's Research Hospital, 332 N. Lauderdale, Memphis, TN 38105, USA
Rights and permissions
About this article
Cite this article
Stewart, C.F., Zamboni, W.C., Crom, W.R. et al. Topoisomerase I interactive drugs in children with cancer. Invest New Drugs 14, 37–47 (1996). https://doi.org/10.1007/BF00173681
Issue Date:
DOI: https://doi.org/10.1007/BF00173681