Abstract
Purpose
Although preclinical studies on camptothecin antitumor effect have demonstrated the superiority of low-dose protracted dosing, these findings were not replicated in the clinic. 7-t-butyldimethylsilyl-10-hydroxycamptothecin (AR-67) is a camptothecin analogue currently under investigation in early phase clinical trials. To maximize the therapeutic potential of AR-67, we sought to identify factors that affect response to treatment.
Methods
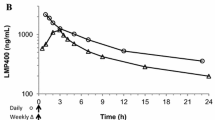
After determining the maximum tolerated dose using neutropenia as a toxicity endpoint, xenografts received AR-67 under varying dosing schedules and were monitored for survival. On the last treatment day, tumor tissue was collected and topoisomerase 1 (Top1), γH2AX, caspase 3 and PARP protein content was evaluated. AR-67 plasma and tumor pharmacokinetics were also studied in mice and cancer patients who were administered AR-67 as a 1-h intravenous infusion on days 1, 4, 8, 12 and 15 every 21 days.
Results
Low-dose protracted dosing schedules increased animal survival compared to less frequent, but higher-dose courses and the expression of Top1 and γH2AX were schedule dependent. Fatigue and neutropenia were the dose-limiting toxicities identified in patients receiving AR-67. Finally, elimination of AR-67 from the tumor site was slower in both xenografts and tumor of a patient enrolled in the pilot clinical trial.
Conclusions
We demonstrated that low-dose protracted dosing schedules of AR-67 are therapeutically effective and Top1 reflects the biological activity of AR-67 in xenografts. Moreover, the tumor pharmacokinetics as well as the efficacy and safety of AR-67 given intermittently to cancer patients warrant further investigation.





Similar content being viewed by others
References
Curran DP, Josien H, Bom D, Gabarda AE, Du W (2000) The cascade radical annulation approach to new analogues of camptothecins. Combinatorial synthesis of silatecans and homosilatecans. Ann N Y Acad Sci 922:112–121
Hsiang YH, Hertzberg R, Hecht S, Liu LF (1985) Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem 260(27):14873–14878
Hsiang YH, Lihou MG, Liu LF (1989) Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res 49(18):5077–5082
Gandia D, Abigerges D, Armand JP, Chabot G, Da Costa L, De Forni M, Mathieu-Boue A, Herait P (1993) CPT-11-induced cholinergic effects in cancer patients. J Clin Oncol 11(1):196–197
Ardizzoni A, Hansen H, Dombernowsky P, Gamucci T, Kaplan S, Postmus P, Giaccone G, Schaefer B, Wanders J, Verweij J (1997) Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J Clin Oncol 15(5):2090–2096
Saliba F, Hagipantelli R, Misset JL, Bastian G, Vassal G, Bonnay M, Herait P, Cote C, Mahjoubi M, Mignard D, Cvitkovic E (1998) Pathophysiology and therapy of irinotecan-induced delayed-onset diarrhea in patients with advanced colorectal cancer: a prospective assessment. J Clin Oncol 16(8):2745–2751
Venditto VJ, Simanek EE (2010) Cancer therapies utilizing the camptothecins: a review of the in vivo literature. Mol Pharm 7(2):307–349. doi:10.1021/mp900243b
Thompson J, Zamboni WC, Cheshire PJ, Lutz L, Luo X, Li Y, Houghton JA, Stewart CF, Houghton PJ (1997) Efficacy of systemic administration of irinotecan against neuroblastoma xenografts. Clin Cancer Res 3(3):423–431
De Cesare M, Pratesi G, Veneroni S, Bergottini R, Zunino F (2004) Efficacy of the novel camptothecin gimatecan against orthotopic and metastatic human tumor xenograft models. Clin Cancer Res 10(21):7357–7364. doi:10.1158/1078-0432.CCR-04-0962
Thompson J, Stewart CF, Houghton PJ (1998) Animal models for studying the action of topoisomerase I targeted drugs. Biochim Biophys Acta 1400(1–3):301–319
Houghton PJ, Cheshire PJ, Hallman JC, Bissery MC, Mathieu-Boue A, Houghton JA (1993) Therapeutic efficacy of the topoisomerase I inhibitor 7-ethyl-10-(4-[1-piperidino]-1-piperidino)-carbonyloxy-camptothecin against human tumor xenografts: lack of cross-resistance in vivo in tumors with acquired resistance to the topoisomerase I inhibitor 9-dimethylaminomethyl-10-hydroxycamptothecin. Cancer Res 53(12):2823–2829
Houghton PJ, Stewart CF, Thompson J, Santana VM, Furman WL, Friedman HS (1998) Extending principles learned in model systems to clinical trials design. Oncology (Williston Park) 12(8 Suppl 6):84–93
Vassal G, Terrier-Lacombe MJ, Bissery MC, Venuat AM, Gyergyay F, Benard J, Morizet J, Boland I, Ardouin P, Bressac-de-Paillerets B, Gouyette A (1996) Therapeutic activity of CPT-11, a DNA-topoisomerase I inhibitor, against peripheral primitive neuroectodermal tumour and neuroblastoma xenografts. Br J Cancer 74(4):537–545
Gerrits CJ, de Jonge MJ, Schellens JH, Stoter G, Verweij J (1997) Topoisomerase I inhibitors: the relevance of prolonged exposure for present clinical development. Br J Cancer 76(7):952–962
Erickson-Miller CL, May RD, Tomaszewski J, Osborn B, Murphy MJ, Page JG, Parchment RE (1997) Differential toxicity of camptothecin, topotecan and 9-aminocamptothecin to human, canine, and murine myeloid progenitors (CFU-GM) in vitro. Cancer Chemother Pharmacol 39(5):467–472
Zamboni WC, Stewart CF, Thompson J, Santana VM, Cheshire PJ, Richmond LB, Luo X, Poquette C, Houghton JA, Houghton PJ (1998) Relationship between topotecan systemic exposure and tumor response in human neuroblastoma xenografts. J Natl Cancer Inst 90(7):505–511
Horn J, Jordan SL, Song L, Roberts MJ, Anderson BD, Leggas M (2006) Validation of an HPLC method for analysis of DB-67 and its water soluble prodrug in mouse plasma. J Chromatogr B Anal Technol Biomed Life Sci 844(1):15–22. doi:10.1016/j.jchromb.2006.06.022
Arnold SM, Rinehart JJ, Tsakalozou E, Eckardt JR, Fields SZ, Shelton BJ, DeSimone PA, Kee BK, Moscow JA, Leggas M (2010) A phase I study of 7-t-butyldimethylsilyl-10-hydroxycamptothecin in adult patients with refractory or metastatic solid malignancies. Clin Cancer Res 16(2):673–680. doi:10.1158/1078-0432.CCR-09-2429
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. doi:10.1016/j.ejca.2008.10.026
Redgate ES, Deutsch M, Boggs SS (1991) Time of death of CNS tumor-bearing rats can be reliably predicted by body weight-loss patterns. Lab Anim Sci 41(3):269–273
Thompson J, Zamboni WC, Cheshire PJ, Richmond L, Luo X, Houghton JA, Stewart CF, Houghton PJ (1997) Efficacy of oral irinotecan against neuroblastoma xenografts. Anticancer Drugs 8(4):313–322
Bence AK, Mattingly CA, Burke TG, Adams VR (2004) The effect of DB-67, a lipophilic camptothecin derivative, on topoisomerase I levels in non-small-cell lung cancer cells. Cancer Chemother Pharmacol 54(4):354–360. doi:10.1007/s00280-004-0804-3
Rasheed ZA, Rubin EH (2003) Mechanisms of resistance to topoisomerase I-targeting drugs. Oncogene 22(47):7296–7304. doi:10.1038/sj.onc.1206935
Furuta T, Takemura H, Liao ZY, Aune GJ, Redon C, Sedelnikova OA, Pilch DR, Rogakou EP, Celeste A, Chen HT, Nussenzweig A, Aladjem MI, Bonner WM, Pommier Y (2003) Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J Biol Chem 278(22):20303–20312. doi:10.1074/jbc.M300198200
Wang LH, Pfister TD, Parchment RE, Kummar S, Rubinstein L, Evrard YA, Gutierrez ME, Murgo AJ, Tomaszewski JE, Doroshow JH, Kinders RJ (2010) Monitoring drug-induced gammaH2AX as a pharmacodynamic biomarker in individual circulating tumor cells. Clin Cancer Res 16(3):1073–1084. doi:10.1158/1078-0432.CCR-09-2799
Huang X, Okafuji M, Traganos F, Luther E, Holden E, Darzynkiewicz Z (2004) Assessment of histone H2AX phosphorylation induced by DNA topoisomerase I and II inhibitors topotecan and mitoxantrone and by the DNA cross-linking agent cisplatin. Cytom A 58(2):99–110. doi:10.1002/cyto.a.20018
Shah GM, Shah RG, Poirier GG (1996) Different cleavage pattern for poly(ADP-ribose) polymerase during necrosis and apoptosis in HL-60 cells. Biochem Biophys Res Commun 229(3):838–844. doi:10.1006/bbrc.1996.1889
Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG (1993) Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res 53(17):3976–3985
Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA et al (1995) Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376(6535):37–43. doi:10.1038/376037a0
Whitacre CM, Zborowska E, Willson JK, Berger NA (1999) Detection of poly(ADP-ribose) polymerase cleavage in response to treatment with topoisomerase I inhibitors: a potential surrogate end point to assess treatment effectiveness. Clin Cancer Res 5(3):665–672
Cairns R, Papandreou I, Denko N (2006) Overcoming physiologic barriers to cancer treatment by molecularly targeting the tumor microenvironment. Mol Cancer Res 4(2):61–70. doi:10.1158/1541-7786.MCR-06-0002
Mayer LD, Janoff AS (2007) Optimizing combination chemotherapy by controlling drug ratios. Mol Interv 7(4):216–223. doi:10.1124/mi.7.4.8
Desai SD, Liu LF, Vazquez-Abad D, D’Arpa P (1997) Ubiquitin-dependent destruction of topoisomerase I is stimulated by the antitumor drug camptothecin. J Biol Chem 272(39):24159–24164
Hochster H, Wadler S, Runowicz C, Liebes L, Cohen H, Wallach R, Sorich J, Taubes B, Speyer J (1999) Activity and pharmacodynamics of 21-day topotecan infusion in patients with ovarian cancer previously treated with platinum-based chemotherapy. J Clin Oncol 17(8):2553–2561
Fujimori A, Harker WG, Kohlhagen G, Hoki Y, Pommier Y (1995) Mutation at the catalytic site of topoisomerase I in CEM/C2, a human leukemia cell line resistant to camptothecin. Cancer Res 55(6):1339–1346
Reckamp KL, Krysan K, Morrow JD, Milne GL, Newman RA, Tucker C, Elashoff RM, Dubinett SM, Figlin RA (2006) A phase I trial to determine the optimal biological dose of celecoxib when combined with erlotinib in advanced non-small cell lung cancer. Clin Cancer Res 12(11 Pt 1):3381–3388. doi:10.1158/1078-0432.CCR-06-0112
Kummar S, Kinders R, Gutierrez ME, Rubinstein L, Parchment RE, Phillips LR, Ji J, Monks A, Low JA, Chen A, Murgo AJ, Collins J, Steinberg SM, Eliopoulos H, Giranda VL, Gordon G, Helman L, Wiltrout R, Tomaszewski JE, Doroshow JH (2009) Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies. J Clin Oncol 27(16):2705–2711. doi:10.1200/JCO.2008.19.7681
Kummar S, Chen A, Ji J, Zhang Y, Reid JM, Ames M, Jia L, Weil M, Speranza G, Murgo AJ, Kinders R, Wang L, Parchment RE, Carter J, Stotler H, Rubinstein L, Hollingshead M, Melillo G, Pommier Y, Bonner W, Tomaszewski JE, Doroshow JH (2011) Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas. Cancer Res 71(17):5626–5634. doi:10.1158/0008-5472.CAN-11-1227
Burke TG, Mishra AK, Wani MC, Wall ME (1993) Lipid bilayer partitioning and stability of camptothecin drugs. Biochemistry 32(20):5352–5364
Gillies RJ, Raghunand N, Karczmar GS, Bhujwalla ZM (2002) MRI of the tumor microenvironment. J Magn Reson Imaging 16(4):430–450. doi:10.1002/jmri.10181
Vaupel P (2004) Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol 14(3):198–206. doi:10.1016/j.semradonc.2004.04.008
Acknowledgments
The authors would like to thank the patients for their participation in this pilot study and Dr. Jamie Horn for sample handling and processing. Moreover, we would like to acknowledge all the health care professionals at the Markey Cancer Center that cared for the study participants. This study was funded by the Markey Cancer Center Pilot Grant and by CA123867.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tsakalozou, E., Adane, E.D., Liang, Y. et al. Protracted dosing of the lipophilic camptothecin analogue AR-67 in non-small cell lung cancer xenografts and humans. Cancer Chemother Pharmacol 74, 45–54 (2014). https://doi.org/10.1007/s00280-014-2472-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2472-2