Abstract
The expression of many genes is altered upon the activation of macrophages by bacterial LPS. These genes play a crucial role in the orchestration of various responses to protect the host against infection. A novel 2.3 kilobase (kb) cDNA, designated IRG1, was obtained from a cDNA library prepared with RNA isolated from RAW 264.7 following lipopolysaccharide stimulation. Sequence analysis of the clone revealed no identity to any known genes but showed the presence of many potential phosphorylation sites suggesting that IRG1 protein product may be regulated at this level. Furthermore, IRG1 contains the motif for glycosaminoglycan attachment site, implying that IRG1 may be a proteoglycan. By interspecific backcross analysis, Irg1 was mapped to mouse chromosome 14 linked to Tyrp2 and Rap2a. The IRG1 message appears 1.5 h following LPS exposure and its induction was not dependent on new protein synthesis. In fact, cycloheximide induced the expression of IRG1 wsuggesting that a protein repressor prevents the expression of IRG1 when uninduced. The role of the protein kinase A pathway in regulating the induction of IRG1 by LPS is questionable, because although forskolin inhibited its induction, neither dibutyrl-cAMP nor 8-(4-chlorophenylthio)-cAMP had much effect on its expression. In contrast, activation of protein kinase C potentiated the LPS response. Chelation of extracellular calcium inhibited IRG1 4 h after LPS induction, while increasing intracellular calcium had little effect on the levels of the IRG1 transcript. Inhibiting tyrosine phosphorylation abrogated the induction of IRG1 by LPS. Hence, the induction of IRG1 by LPS is mediated by tyrosine kinase and protein kinase C pathway.
Similar content being viewed by others
References
Adams, D. O. and Hamilton, T. A. The cell biology of macrophage activation. Annu Rev Immunol 2: 283–318, 1984
Adams, D. O. and Hamilton, T. A. Activation of macrophages for tumor cell kill: effector mechanisms and regulation. In G. H. Heppner and A. M. Fulton (eds.): Macrophages and Cancer, pp. 28–35, CRC Press, Baco Raton, 1988
Adams, D. O. and Hamilton, T. A. Macrophages as destructive cells in host defense. In J. I. Gallin, I. M. Goldstein, and R. Snyderman (eds.): Inflammation: Basic Principles and Clinical Correlates, pp. 637–662, Raven Press Ltd., New York, 1992
Akiyama, T., Ishida, J., Nakagawa, S., Ogawara, H., Watanabe, S., Itoh, N., Shibuya, M., and Fukami, Y. Genestein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262: 5592–5596, 1987
Anttila, H. S., Reitamo, S., Ceska, M., and Hurme, M. Signal transduction pathways leading to the production of IL-8 by human monocytes are differentially regulated by dexamethasone. Clin Exp Immunol 89: 509–512, 1992
Asaoka, Y., Nakamura, S., Yoshida, K., and Nishizuka, Y. Protein kinase C, calcium and phospholipid degradation. Trends Biochem Sci 17: 414–417, 1992
Benveniste, E. N., Sparacio, S. M., Norris, J. G., Grenett, H. E., and Fuller, G. M. Induction and regulation of interleukin 6 gene expression in rat astrocytes. J Neuroimmunol 30: 201–212, 1990
Berridge, M. J. Inositol triphosphate and diacyglycerol as second messengers. Biochem J 220: 345–360, 1984
Berry, L. J. and Smyth, D. S. Effects of bacterial endotoxins on metabolism. VII. Enzyme induction and cortisone protection. J Exp Med 120: 721–732, 1984
Bravo, R., Neuberg, M., Burckhardt, J., Almendral, J., Wallich, R., and Muller, R. Involvement of common and cell type-specific pathways in c-fos gene control: stable induction by cAMP in macrophages. Cell 48: 251–260, 1987
Buchmuller-Rouiller, Y. and Mauel, J. Macrophage activation for intracellular killing as induced by calcium ionophore. J Immunol 146: 217–223, 1991
Cantley, L. C., Auger, K. R., Carpenter, C., Duckworth, B., Graziani, A., Kapeller, R., and Soltoff, S. Oncogenes and signal transduction. Cell 164: 281–302, 1991
Caput, D., Beutler, B., Hartog, K., Thayer, R., Brown-Shimer, S., and Cerami, A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci USA 83: 1670–1674, 1986
Coffey, R. G. and Hadden, J. W. Cyclic nucleotides and lipopolysacchride action. In D. C. Morrison and J. L. Ryan (eds.): Bacterial Endotoxic Lipopolysacchrides, pp. 3–26, CRC Press, Boca Raton, 1992
Cook, S. J. and Wakelam, M. J. O. Phospholipase C and D in mitogenic signal transduction. Rev Physiol Biochem Pharmacol 119: 13–45, 1992
Copeland, N. G. and Jenkins, N. A. Development and applications of a molecular genetic linkage map of the mouse genome. Trends Genet 7: 113–118, 1991
Crossman, D. C., Carr, D. P., Tuddenham, E. G., Pearson, J. D., and McVey, H. H. The regulation of tissue factor mRNA in human endothelial cells in response to endotoxin or phorbol ester. J Biol Chem 265: 9782–9787, 1990
Debets, J. M. H., Ruers, T. J. M., and Van der Linden, M. P. Inhibitory effect of corticosteroids on the secretion of tumor necrosis factor (TNF) by monocytes is dependent on the stimulus inducing TNF synthesis. Clin Exp Immunol 78: 224–229, 1989
Dong, Z., O'Brian, C. A., and Fidler, I. J. Activation of tumoricidal properties in macrophages by lipopolysaccharides require protein tyrosine kinase activity. J Leuk Biol 53: 53–60, 1993 a
Dong, Z., Qi, X., Xie, K., and Fidler, I. J. Protein tyrosine kinase inhibitors decrease induction of nitric oxide synthase activity in lipopolysaccharide-responsive and lipopolysaccharide-nonresponsive murine macrophages. J Immunol 151: 2717–2724, 1993 b
Drysdale, B., Yapundich, R. A., Shin, M. L., and Shin, H. S. Lipopolysaccharide-mediated macrophage activation: the role of calcium in the generation of tumoricidal activity. J Immunol 38: 951–956, 1987
Fan, X. D., Stark, G. R., and Bloom, B. R. Molecular cloning of a gene selectively induced by λ interferon from human macrophage cell line U937. Mol Cell Biol 9: 1922–1928, 1989
Fidler, I. J. Macrophages and metastasis — a biological approach to cancer therapy: Presidential address. Cancer Res 45: 4714–4726, 1985
Frohman, M. A. RACE: Rapid amplification of cDNA ends. In M. A. Innis, D. H. Gelfrand, J. J. Sninsky, and T. J. White (eds.): PCR Protocols. A Guide to Methods and Applications, pp. 28–38, Academic Press, San Diego, 1990
Gardner, P. Patch clamp studies of lymphocyte activation. Annu Rev Immunol 8: 231–252, 1990
Ghezzi, P. and Sipe, J. D. Dexamethasone modulation of LPS, IL-1, and TNF stimulated serum amyloid A synthesis in mice. Lymphocine Res 7: 157–166, 1988
Gorecka-Tisera, A. M., Snowdowne, K. W., and Borle, A. B. Implications of a rise in cytosolic free calcium in the activation of RAW-264 macrophages for tumor cell killing. Cell Immunol 100: 411–421, 1986
Green, E. L. Genetics and Probability in Animal Breeding Experiments, pp. 77–113, Oxford University Press, New York, 1981
Gutch, M. J., Daly, C., and Reich, N. C. Tyrosine phosphorylation is required for activation of an α interferon-stimulated transcription factor. Proc Natl Acad Sci USA 89: 11411–11415, 1992
Hall, D. J., and Stiles, C. D. Platelet-derived growth factor-inducible genes respond differentially to at least two distinct intracellular second messengers. J Biol Chem 262: 15302–15308, 1987
Hamilton, J. A., Veis, N., Bordun, A., Vairo, G., Gonda, J. J., and Philips, W. A. Activation and proliferation signals in murine macrophages: relationships among c-fos and c-myc expression, phosphoinositide hydrolysis, superoxide formation, and DNA synthesis. J Cell Physiol 141: 618–626, 1989
Hamilton, J. A., Whitty, G. A., Last, K., Royston, A. K., Hart, P. H., and Burgens, D. R. Interleukin-4 suppresses plasminogen activator inhibitor-2 formation in stimulated human monocytes. Blood 80: 121–125, 1992
Hamilton, T. A., Bredon, N., Ohmori, Y., and Tannenbaum, C. S. IFN-λ and IFN-β independently stimulate the expression of lipopolysaccharide-inducible genes in murine peritoneal macrophages. J Immunol 142: 2325–2331, 1989
Hamilton, T. A., Ohmori, Y., Narumi, S., and Tannenbaum, C. S. Regulation of Diversity of Macrophage Activation. In G. Lopez-Berestein and J. Klostergaard (eds.): Mononuclear Phagocytes in Cell Biology, pp. 48–65, CRC Press, Ann Arbor, 1993
Hamilton, T. A., Ohmori, Y., Tebo, J. M., Narumi, S., and Tannenbaum, C. S. Transmembrane and intracellular signaling events in lipopolysaccharide-stimulated macrophages. In B. S. Zwilling and T. K. Eisenstein (eds.): Macrophage — Pathogen Interactions, pp. 83–97, Marcel Dekker, New York, 1994
Hidaka, H., Inagaki, M., Kawamoto, S., and Sasaki, Y. Isoquinoline-sulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry 23: 5036–5041, 1984
Hurme, M., Siljander, P., and Anttila, H. Regulation of interleukin-1 β production by glucocorticoids in human monocytes: the mechanism of action depends on the activation signal. Biochem Biophys Res Commun 180: 1383–1389, 1991
Hurme, M. and Serkkola, E. Different activation signals are required for the expression of interleukin-1 α and β genes in human monocytes. Scand J Immunol 33: 713–718, 1991
Jackson, I. J., Chambers, D. M., Tsukamoto, K., Copeland, N. G., Gilbert, D. J., Jenkins, N. A., and Hearing, V. A second tyrosinase-related protein, TRP-2, maps to and is mutated at the mouse slaty locus. EMBO J 11: 527–535, 1992
Jenkins, N. A., Copeland, N. G., Taylor, B. A., and Lee, B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus Musculus. J Virol 43: 26–36, 1982
Kammer, G. M. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol Today 9: 222–229, 1988
Kovacs, E. J. Control of IL-1 and TNFα production at the level of second messenger pathways. In E. S. Kimball (ed.): Cytokines and Inflammation, pp. 89–107, CRC Press, Boca Raton, 1991
Kruys, V., Wathelet, M., Poupart, P., Contreras, R., Fiers, W., Content, J., and Huez, G. The 3′ untranslated region of the human interferon-β mRNA has an inhibitory effect on translation. Proc Natl Acad Sci USA 84: 6030–6034, 1987
Kruys, V. I., Wathelet, M. G., and Huez, G. A. Identification of a translation inhibitory element (TIE) in the 3′ unstranlated region on human interferon β mRNA. Gene 72: 191–200, 1988
Lee, C. G. L., Demarquoy, J., Jackson, M. J., and O'Brien, W. E. Molecular cloning and characterization of a murine LPS-inducible cDNA. J Immunol 152: 5758–5767, 1994
Letari, O., Nicosia, S., Chiavaroli, C., Vacher, P., and Schlegel, W. Activation by bacterial lipopolysaccharide causes changes in the cytosolic free calcium concentration in single peritoneal macrophages. J Immunol 147: 980–983, 1991
MacIntyre, J. P. and Pope, B. L. The involvement of protein kinase C, calcium and 5-lipoxygenase in the production of tumor necrosis factor by a cloned interleukin 3 dependent cell line with natural cytotoxic activity. Int J Immunopharmacol 13: 175–184, 1991
Mahe, Y., Wakasugi, H., Scamps, C., Chouaib, S., and Tursz, T. Role of calcium on interleukin 1 production by monocytes: its relevance during T cell proliferation. Lymphokine Cytokine Res 10: 165–172, 1991
Martin, C. A. and Dorf, M. E. Differential regulation of interleukin-6, macrophage inflammatory protein-1, and JE/MCP-1 cytokine expression in macrophage cell lines. Cell Immunol 135: 245–258, 1991
McCallum, R. E., Seale, T. W., and Stith, R. D. Influence of endotoxin treatment on dexamethasone induction of hepatic phosphoenolpyruvate carboxykinase. Infect Immunol 39: 213–219, 1983
Ohmori, Y., Strassman, G., and Hamilton, T. A. cAMP differentially regulates expression of mRNA encoding IL-1 α and IL-1 β in murine peritoneal macrophages. J Immunol 145: 3333–3339, 1990
Oka, S. and Arita, H. Inflammatory factors stimulate expression of group II phospholipase A2 in rat cultured astrocytes. J Biol Chem 266: 9956–9960, 1991
Pawson, T. Tyrosine kinases and their interactions with signalling protein. Curr Opin Genet Dev 2: 4–12, 1992
Pizon, V., Chardin, P., Lerosey, I., Olofsson, B., and Tavitian, A. Human cDNAs rap1 and rap2 homologous to the Drosophila gene Dras3 encode proteins closely related to ras in the “effector” region. Oncogene 3: 201–204, 1988
Price, K. H., Choi, H. U., Rosenberg, L., and Stanley, E. R. The predominant form of secreted colony stimulating factor-1 is a proteoglycan. J Biol Chem 267: 2190–2199, 1992
Renauld, J. C., Goethals, A., Houssiau, F., Merz, H., Van Roost, E., and Van Snick, J. Human P40/IL-9. Expression in activated CD4+ T cells, genomic organization, and comparison with the mouse gene. J Immunol 144: 4235–4241, 1990
Rhee, S. G. Inositol phospholipid-specific phospholipase C: interaction of γ1 isoform with tyrosine kinase. Trends Biochem Sci 16: 297–301, 1991
Seamon, K. B., Padgett, W., and Daly, J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci USA 78: 3363–3367, 1981
Shaw, G. and Kamen, R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell 46: 659–667, 1986
Shen, X. Y., Hamilton, T. A., and Dicorleto, P. E. Lipopolysaccharide-induced expression of the competence gene KC in vascular endothelial cells is mediated through protein kinase C. J Cell Physiol 140: 44–51, 1989
Shurtleff, S. A., McElwain, C. M., and Taffet, S. M. Rapid expression of ornithine decarboxylase mRNA in a macrophage-like cell line: cAMP regression of the requirement for prior protein synthesis. J Cell Physiol 134: 453–459, 1988
Shyy, Y. J., Li, Y. S., and Kolattukudy, P. E. Activation of MCP-1 gene expression is mediated through multiple signalling pathways. Biochem Biophys Res Commun 192: 693–699, 1993
Simon, M. I., Strathman, M. P., and Gautam, N. Diversity of G proteins in signal transduction. Science 252: 802–808, 1991
Slivka, S. R. and Loskutoff, D. J. Regulation of type I plasminogen activator inhibitor synthesis by protein kinase C and cAMP in bovine aortic endothelial cells. Biochim Biophys Acta 1094: 317–322, 1991
Smith, J. M. F., Kueppers, F., and Lee, J. C. Differential regulation of interleukin-1 α and interleukin-1 β mRNA expression in human monocytes: evidence for protein kinase C-dependent and independent pathways. Lymphokine Cytokine Res 10: 397–403, 1991
Spengler, R. N., Spengler, M. L., Lincoln, P., Remick, D. G., Strieter, R. M., and Kunkel, S. L. Dynamics of dibutyryl cyclic AMP-and prostaglandin E2-mediated suppression of lipopolysaccharide-induced tumor necrosis factor α gene expression. Infect Immun 57: 2837–2841, 1989
Sung, S. S. and Walters, J. A. Increased cyclic AMP levels enhance IL-1 α and IL-1 β mRNA expression and protein production in human myelomonocytic cell lines and monocytes. J Clin Invest 88: 1915–1923, 1991
Tanaka, Y., Adams, D. H., Hubscher, S., Hirano, H., Siebenlist, U., and Shaw, S. T-cell adhesion induced by proteoglycam-imobilized cytokine MIP-1β. Nature 361: 79–82, 1993 a
Tanaka, Y., Adams, D. H., and Shaw, S. Proteoglycans on endothelial cells present adhesion-inducing cytokines to leukocytes. Immunol Today 14: 111–115, 1993 b
Tannenbaum, C. S., Koerner, T. J., Jansen, M. M., and Hamilton, T. A. Characterization of lipopolysaccharide-induced macrophage gene expression. J Immunol 140: 3640–3645, 1988
Tannenbaum, C. S. and Hamilton, T. A. Lipopolysaccharide-induced gene expression in murine peritoneal macrophages is selectively suppressed by agents that elevate intracellular cAMP. J Immunol 142: 1274–1280, 1989
Van Halen, F. and Keck, E. Forskolin inhibition of glucose transport in bone cell cultures through a cAMP-independent mechanism. Bone 9: 89–92, 1888
Van Ranst, M., Norga, K., Masure, S., Proost, P., Vandekerckhove, F., Auwerx, J., Van Damme, J., and Opdenakker, G. The cytokine-protease connection: identification of a 96-kD THP-1 gelatinase and regulation by interleukin-1 and cytokine inducers. Cytokine 3: 231–239, 1991
Wagner, P. K. and Palotta, B. S. Modulation of acetylcholine receptor desensitization by forskolin is independent of cAMP. Science 240: 1655–1657, 1988
Weinstein, S. L., Gold, M. R., and DeFranco, A. L. Bacterial lipopolysaccharide stimulates protein tyrosine phosphorylation in macrophages. Proc Natl Acad Sci USA 88: 4148–4152, 1991
Weinstein, S. L., June, C. H., and DeFranco, A. L. Lipopolysaccharide-induced protein tyrosine phosphorylation in human macrophages is mediated by CD14. J Immunol 151: 3829–3838, 1993
White, M. M. Forskolin alters acetylcholine receptor gating by a mechanism independent of cAMP. Mol Pharmacol 34: 427–430, 1988
Wightman, P. D. and Raetz, C. R. H. The activation of protein kinase C by the lipid moieties of lipopolysaccharide. Fed Proc 45: A1936, 1986
Wright, B., Zeidman, I., Greig, R., and Poste, G. Inhibition of macrophage activation by calcium channel blockers and calmodulin antagonists. Cell Immunol 95: 46–53, 1985
Zuenkler, B. J., Trube, G., and Ohno-Shosaku, T. Forskolin-induced block of delayed rectifying potassium channels in pancreatic β-cells is not mediated by cAMP. Pfluegers Arch 411: 613–619, 1988
Author information
Authors and Affiliations
Additional information
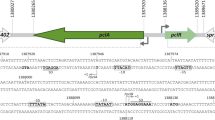
The nucleotide sequence data reported in this paper have been submitted to the GenBank nucleotide sequence database and have been assigned the accession number L38281
Rights and permissions
About this article
Cite this article
Lee, C.G.L., Jenkins, N.A., Gilbert, D.J. et al. Cloning and analysis of gene regulation of a novel LPS-inducible cDNA. Immunogenetics 41, 263–270 (1995). https://doi.org/10.1007/BF00172150
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00172150