Summary
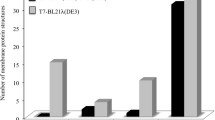
Membranes of Escherichia coli cells grown in the presence of phenol were examined after isolation of the cytoplasmic and outer membrane fractions. Both membrane types showed reduced lipid-to-protein ratios compared to cells grown without phenol. Phenol-induced differences in the expression of individual proteins of the outer membrane, probably involved in the uptake of iron, were expressed in smaller quantities after phenol addition. Growth in the presence of phenol increased the respiratory activity of the cytoplasmic membrane, whereas the direct inhibition of O2 consumption by phenol was not affected by the presence of this compound in the growth medium. E. coli cells grown entrapped in calcium alginate showed low lipid-to-protein ratios even without phenol in the growth medium. Immobilization of cells also markedly changed the protein pattern of the outer membrane.
Similar content being viewed by others
References
Bettmann H, Rehm HJ (1984) Degradation of phenol by polymer entrapped microorganisms. Appl Microbiol Biotechnol 20:285–290
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Davidson PM, Branen AL (1981) Antimicrobial activity of nonhalogenated phenolic compounds. J Food Prot 44:623–632
Dombek KM, Ingram LO (1984) Effects of ethanol on the Escherichia coli plasma membrane. J Bacteriol 157:233–239
Eikmeier H, Rehm HJ (1987) Stability of calcium-alginate during citric acid production of immobilized Aspergillus niger. Appl Microbiol Biotechnol 26:105–111
Fiske CH, Subarrow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Hamilton WA (1971) Membrane active antibacterial compounds. In: Hugo WB (ed) Inhibition and destruction of the microbial cell. Academic Press, London, pp 77–93
Herring FG, Krisman A, Sedgwick EG, Bragg PD (1985) Electron spin resonance studies of lipid fuidity changes in membranes of an uncoupler-resistant mutant of Escherichia coli. Biochim Biophys Acta 819:231–240
Hugo WB (1978) Membrane-active antimicrobial drugs — a reappraisal of their mode of action in the light of the chemiosmotic theory. Int J Pharm 1:127–131
Jähnig F (1979) Structural order of lipids and proteins in membranes: evaluation of fluorescence anisotropy data. Proc Natl Acad Sci USA 76:6361–6365
Jones NC, Osborn MJ (1977) Translocation of phospholipids between the outer and inner membranes of Salmonella typhimurium. J Biol Chem 252:7405–7412
Keweloh H, Heipieper HJ, Rehm HJ (1989a) Protection of bacteria against toxicity of phenol by immobilization in calcium alginate. Appl Microbiol Biotechnol 31:383–389
Keweloh H, Heipieper HJ, Rehm HJ (1989b) Tolerance of bacteria against phenol after immobilization in Ca-alginate. In: Dechema — Biotechnol Conferences, vol. 3. VCH Weinheim, in press
Klebba PE, McIntosh MA, Neilands JB (1982) Kinetics of biosynthesis of iron-regulated membrane proteins in Escherichia coli. J Bacteriol 149:880–888
LeChevallier MW, Cawthon CD, Lee RG (1988) Inactivation of biofilm bacteria. Appl Environ Microbiol 54:2492–2499
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lugtenberg B, Meijers J, Peters R, Hoek P van der, Alphen L van (1975) Electrophoretic resolution of the major outer membrane protein of Escherichia coli K 12 into four bands. FEBS Lett 58:254–258
Lugtenberg B, Peters R, Bernheimer H, Berendsen W (1976) Influence of cultural conditions and mutations on the composition of the outer membrane proteins of Escherichia coli. Mol Gen Genet 147:251–262
Markwell MK, Haas SM, Bieber LL, Tolbert NE (1978) A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 87:206–210
Mattiasson B, Larsson M, Hahn-Hägerdal B (1984) Metabolic behavior of immobilized cells — effects of some microenvironmental factors. Ann NY Acad Sci Enz Eng 7:475–478
McIntosh MA, Earhart CF (1977) Coordinate regulation by iron of the synthesis of phenolate compounds and three outer membrane proteins in Escherichia coli. J Bacteriol 131:331–339
McIntyre TM, Bell RM (1975) Mutants of Escherichia coli defective in membrane phospholipid synthesis. Effect of cessation of net phospholipid synthesis on cytoplasmic and outer membranes. J Biol Chem 250:9053–9059
Miller CG (1987) Protein degradation and proteolytic modification. In: Neidhardt FC (ed) Escherichia coli and Salmonella typhimurium. ASM, Washington, pp 680–691
Nichols WW, Evans MJ, Slack MPE, Walmsley HL (1989) The penetration of antibiotics into aggregates of mucoid and nonmucoid Pseudomonas aeruginosa. J Gen Microbiol 135:1291–1303
Nunn WD, Cheng P, Deutsch R, Tang CT, Tropp BE (1977) Phenethyl alcohol inhibition of sn-glycerol 3-phosphate acylation in Escherichia coli. J Bacteriol 130:620–628
Oakley BR, Kirsch DR, Morris NR (1980) A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem 105:361–363
Osborn MJ, Gander JE, Parisi E, Carson J (1972) Mechanism of assembly of the outer membrane of Salmonella typhimurium. J Biol Chem 247:3962–3972
Osman YA, Ingram LO (1985) Mechanism of ethanol inhibition of fermentation in Zymomonas mobilis CP4. J Bacteriol 164:173–180
Papahadjopoulos D, Vail WJ, Newton C, Nir S, Jacobson K, Poste G, Lazo R (1977) Studies on membrane fusion. III The role of calcium-induced phase changes. Biochim Biophys Acta 465:579–598
Salgueiro SP, Sa-Correia I, Novais JM (1988) Ethanol-induced leakage in Saccharomyces cerevisiae: kinetics and relationship to yeast ethanol tolerance and alcohol fermentation productivity. Appl Environ Microbiol 54:903–909
Westmeier F, Rehm HJ (1985) Biodegradation of 4-chlorophenol by entrapped Alcaligenes sp. A 7–2. Appl Microbiol Biotechnol 22:301–305
Weyrauch G (1989) Einfluß von Phenol auf die Membranen freier und immobilisierter Zellen von Escherichia coli. Diploma thesis, Institute for Microbiology, University of Münster
Author information
Authors and Affiliations
Additional information
Offprint requests to: H.-J. Rehm
Rights and permissions
About this article
Cite this article
Keweloh, H., Weyrauch, G. & Rehm, HJ. Phenol-induced membrane changes in free and immobilized Escherichia coli . Appl Microbiol Biotechnol 33, 66–71 (1990). https://doi.org/10.1007/BF00170572
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00170572