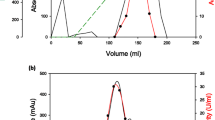
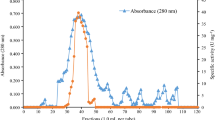
Abstract
An alkalophilic strain of Bacillus sp., designated TS-23, was isolated from a soil sample collected at a hot spring (Tainan, Taiwan). During growth in a medium containing 1% soluble starch as the sole source of carbon, the fermentation broth exhibited both pullulanase and amylase activity. Pullulanase and amylase activities were maximal at 65° C. The pH optima were 8.8 to 9.6 for pullulanase and 7.5 to 9.4 for amylase. Under optimal conditions, a crude preparation hydrolysed pullulan, generating maltotriose as the major product. Strain TS-23 was found to produce five amylases (Ac, A1, A2, AP1, and AP2), which were visualized by activity staining of proteins that had been separated by native polyacrylamide gel electrophoresis. Both AP1 and AP2 had pullulanase activity and Ac, A1 and A2 had the ability to adsorb to raw corn-starch. Native corn-starch was partially digested by adsorbed amylases during the course of 12 h at 50° C, with initiation of granular pitting. Further incubation of the reaction mixture resulted in considerable morphological changes in corn-starch granules, and the main soluble products were maltose, maltotriose and higher oligosaccharides.
Similar content being viewed by others
References
Abdullah M, French, D (1970) Substrate specificity of pullulanase. Arch Biochem Biophys 137:483–493
Brown SH, Kelly RM (1993) Characterization of amylolytic enzymes, having both α-1,4 and α-1,6 hydrolytic activity, from the thermophilic archaea Pyrococcus furiosus and Thermococcus litoralis. Appl Environ Microbiol 59:2614–2621
Brown SH, Costantino HR, Kelly RM (1990) Characterization of amylolytic enzyme activities associated with the hyperthermophilic archaebacterium Pyrococcus furiosus. Appl Environ Microbiol 56:1985–1991
Buranakarl L, Ito K, Izaki K, Takahashi H (1988) Purification and characterization of a raw starch-digestive amylase from nonsulfur purple photosynthetic bacterium. Enzyme Microb Technol 10:173–179
Claus D, Berkeley RCW (1986) Genus Bacillus. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG (eds) Bergey's manual of systematic bacteriology. Williams & Wilkins, Baltimore, pp 1105–1139
Colman RD, Yang SS, McAlister MP (1987) Cloning of the debranching-enzyme gene from Thermoanaerobium brockii into Escherichia coli and Bacillus subtilis. J Bacteriol 169:4302–4307
Costantino HR, Brown SH, Kelly RM (1990) Purification and characterization of an α-glucosidase from a hyperthermophilic archaebacterium, Pyrococcus furiosus, exhibiting a temperature optimum of 105 to 115° C. J Bacteriol 172:3654–3660
Fang TY, Lin LL, Hsu WH (1994) Recovery of isoamylase from Pseudomonas amyloderamosa by adsorption-elution on raw starch. Enzyme Microb Technol 16:247–252
Fogarty WM, Kelly CT (1979) Starch-degrading enzymes from microbial origin. Prog Ind Microbiol 15:87–150
Fogarty WM, Kelly CT (1980) Amylases, amyloglucosidases and related glucanases. In: Rose AH (ed) Microbial enzymes and bioconversions. Academic Press, New York, pp 115–169
Hayashida S, Teramoto T, Inoue T (1988) Production and characteristics of raw-potato-starch digesting α-amylase from Bacillus subtilis 65. Appl Environ Microbiol 54:1516–1522
Kim CH, Kim DS, Taniguchi H, Maruyama Y (1990) Purification of an amylase-pullulanase bifunctional enzyme by high performance size-exclusion and hydrophobic-interaction chromatography. J Chromatogr 512:131–137
Koch R, Spreinat A, Lemke K, Antranikian G (1991) Purification and properties of a hyperthermoactive α-amylase from the archaebacterium Pyrococcus furiosus. Arch Microbiol 155:572–578
Mathupala S, Saha BC, Zeikus JG (1990) Substrate competition and specificity at the active site of amylopullulanase from Clostridium thermohydrosulfuricum. Biochem Biophys Res Commun 166:126–132
Melasiemi H (1987) Characterization of α-amylase and pullulanase activities of Clostridium thermohydrosulfuricum. Biochem J 246:193–197
Melasiemi H (1988) Purification and some properties of the extracellular α-amylase-pullulanase produced by Clostridium thermohydrosulfuricum. Biochem J 250:813–818
Miller GL (1959) Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Morgan FJ, Adams KR, Priest FG (1979) A cultural method for the detection of pullulan-degrading enzymes in bacteria and its application to the genus Bacillus. J Appl Bacteriol 46:291–294
Norman BE (1979) The application of polysaccharide degrading enzymes in the starch industry. In: Berkeley RCW, Gooday GW, Ellwood DC (eds) Microbial polysaccharides and polysaccharases. Academic Press, New York, pp 339–376
Plant AR, Clemens RM, Morgan HW, Daniel RM (1987) Active site- and substrate-specificity of Thermoanaerobium Tok6-B1 pullulanase. Biochem J 246:537–541
Saha BC, Zeikus JG (1989) Novel highly thermostable pullulanase from thermophiles. Trends Biotechnol 7:234–239
Saha BC, Mathupala SP, Zeikus JG (1988) Purification and characterization of a highly thermostable novel pullulanase from Clostridium thermohydrosulfuricum. Biochem J 252:343–348
Saha BC, Shen GJ, Srivastava KC, LeCureux LW, Zeikus JG (1989) New thermostable α-amylase-like pullulanase from thermophilic Bacillus sp. 3183. Enzyme Microb Technol 11:760–764
Shen GJ, Crivastava KC, Saha BC, Zeikus JG (1990) Physiological and enzymatic characterization of a novel pullulan-degrading thermophilic Bacillus strain 3183. Appl Microbiol Biotechnol 33:340–344
Spreinat A, Antranikian G (1992) Analysis of the amylolytic enzyme system of Clostridium thermosulfurogenes EM1: purification and synergistic action of pullulanase and maltohexaose forming α-amylase. Starch/Stärke 44:305–312
Sreenath HK, Lafayette W (1992) Studies on starch granules digestion by α-amylase. Starch/Stärke 44:61–63
Takasaki Y (1987) Pullulanase-amylase complex enzyme from Bacillus subtilis. Agric Biol Chem 51:9–16
Tanaka T, Ishimoto E, Shimomura Y, Taniguchi M (1987) Purification and some properties of raw starch-binding amylase of Clostridium butyricum T7 isolated from mesophilic methane sludge. Agric Biol Chem 51:399–405
Taniguchi H, Odashima F, Igarashi M, Maruyama Y, Nakamura M (1982) Characterization of a potato starch-digesting bacterium and its production of amylase. Agric Biol Chem 46:2107–2115
Taniguchi H, Jae CM, Yoshigi N, Maruyama Y (1983) Purification of Bacillus circulans F-2 amylase and its general properties. Agric Biol Chem 47:511–519
Yoshigi N, Chikano T, Kamimura M (1985) Purification and properties of an amylase from Bacillus cereus NY-14. Agric Biol Chem 49:3369–3376
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lin, LL., Tsau, MR. & Chu, WS. General characteristics of thermostable amylopullulanases and amylases from the alkalophilic Bacillus sp. TS-23. Appl Microbiol Biotechnol 42, 51–56 (1994). https://doi.org/10.1007/BF00170224
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00170224