Abstract
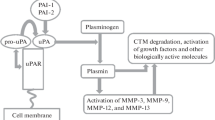
We have investigated the effect of the immunomodulator ubenimex (hereafter referred to as bestatin) on the enzymatic degradation of the extracellular matrix by human renal cell carcinoma SN12M cells during the invasive process. The invasion of SN12M cells into reconstituted basement membrane (Matrigel) was inhibited by the presence of bestatin in a concentration-dependent manner. However, bestatin did not have any effect on tumor cell adhesion and migration to the extracellular matrices which may be involved in tumor cell invasion. Bestatin inhibited the degradation of type IV collagen by tumor cells, but not by tumor-conditioned medium (TCM), in a concentration-dependent manner. We also found that bestatin inhibited hydrolysing activities towards substrates of aminopeptidases in SN12M cells. Since bestatin was found to inhibit aminopeptidase activity, the inhibition of tumor invasion by bestatin is likely to be associated with its action as an enzyme inhibitor. Bestatin only slightly inhibited tumor cell plasmin activity, which can lead to the conversion of the latent collagenase to the active form, but this slight effect was not significant. The zymography of TCM from SN12M cells showed that the treatment of tumor cells with bestatin resulted in the disappearance of the 68 kDa type IV collagenase-enzyme level (active form) and slight reduction of the 72 kDa type IV collagenase-enzyme level (latent form). These results indicated that bestatin may inhibit tumor cell invasion through a mechanism involving its inhibitory action on aminopeptidases in tumor cells, suggesting that the aminopeptidase may partly be associated with the conversion of a latent form of type IV procollagenase to an active form or the secretion of the collagenases from tumor cells.
Similar content being viewed by others
References
Fidler IJ, 1990, Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes Memorial Award Lecture. Cancer Research, 50, 6130–6138.
Fidler IJ, Gersten DM and Hart IR, 1978, The biology of cancer invasion and metastases. Advances in Cancer Research, 28, 149–250.
Liotta LA, Rao CV and Barsky SH, 1983, Tumor invasion and the extracellular matrix. Laboratory Investigation, 49, 639–649.
McCarthy JB, Basara ML, Palm SL, Sas DF and Furcht LT, 1985, The role of cell adhesion proteins-laminin and fibronectin—in the movement of malignant and metastatic cells. Cancer and Metastasis Reviews, 4, 125–152.
Moscatelli D and Rifkin DB, 1988, Membrane and matrix localization of proteinases—a common theme in tumor cell invasion and angiogenesis. Biochimica et Biophysica Acta, 948, 67–85.
Nicolson GL, 1987, Tumor cell instability, diversification, and progression to the metastatic phenotype: from oncogene to oncofetal expression. Cancer Research, 47, 1473–1487.
Poste G and Fidler IJ, 1979, The pathogenesis of cancer metastasis. Nature, 283, 139–146.
Strauli P and Haemmerli G, 1984, The role of cell motility in invasion. Cancer and Metastasis Reviews, 3, 127–141.
Tryggvason K, Hoyhtya M and Salo T, 1987, Proteolytic degradation of extracellular matrix in tumor invasion. Biochimica et Biophysica Acta, 907, 191–217.
Weiss L, 1985, Principles of Metastasis (Orlando: Academic Press).
Kuramochi H, Motegi A, Iwabuchi M, Takahashi K, Horinishi H and Umezawa H, 1987, Action of ubenimex on aminopeptidase activities in spleen cells and peritoneal macrophages from mice. Journal of Antibiotics, 40, 1605–1611.
Leyhausen G, Schuster DK, Vaith P, Zahn RK, Umezawa H, Falke D and Muller WEG, 1983, Identification and properties of the cell membrane bound leucine aminopeptidase interacting with potential immunostimulant and chemotherapeutic agent bestatin. Biochemical Pharmacology, 32, 1051–1057.
Suda H, Aoyagi T, Takeuchi T and Umezawa H, 1976, Inhibition of aminopeptidase B and leucine aminopeptidase by bestatin and its stereoisomers. Archives of Biochemistry and Biophysics, 177, 196–200.
Abe F, Shibuya K, Uchida M, Takahashi K, Horinishi H, Matsuda A, Ishizuka M, Takeuchi T and Umezawa H, 1984, Effect of bestatin on syngeneic tumors in mice. Gann, 75, 89–94.
Ikeda S, Miyasato H, Saito K, Nakayama H and Tajima K, 1981, Phase I study of bestatin, (II) clinical trial of bestatin in malignant skin tumors. In Small Molecular Immunomodifiers of Microbial Origin, edited by H. Umezawa (New York: Pergamon Press), pp. 143–158.
Ishizuka M, Masuda T, Kanbayashi N, Fukusawa S, Takeuchi T, Aoyagi T and Umezawa H, 1980, Effect of bestatin on mouse immune system and experimental murine tumors. Journal of Antibiotics, 33, 642–652
Majima H, 1981, Phase I and preliminary phase II clinical trials of bestatin. In Small Molecular Immunomodifiers of Microbial Origin, edited by H. Umezawa (New York: Pergamon Press), pp. 173–178.
Miwa H, Oka T, Tsurumi T, Sakae Y and Orita K, 1982, Experimental and clinical studies of bestatin as an immunomodulator. Japanese Journal of Cancer Chemotherapy, 9, 1019–1024.
Schlorlemmer H-U, Bosslet K, Dickneite G, Luben G and Sedlacek HH, 1984, Studies on the mechanisms of action of the immunomodulator bestatin in various screening test systems. Behring Institute Mitteilung, 74, 157–173.
Bruley-Rosset M, Florentin I, Kiger N, Schulz J and Mathe G, 1979, Restoration of impaired immune functions of aged animals by chronic bestatin treatment. Immunology, 38, 75–83.
Dunlap BE, Dunlap SA and Rich DH, 1984, Effect of bestatin on in vitro responses of murine lymphocytes to T-cell stimuli. Scandinavian Journal of Immunology, 20, 237–245.
Saito K, Takegoshi K, Aoyagi T, Umezawa H and Nagai Y, 1978, Stimulatory effect of bestatin, a new specific inhibitor of aminopeptidase, on the blasto genesis of guinea pig lymphocytes. Cellular Immunology, 40, 242–262.
Schoremmer H-U, Bosslet K and Sedlacek HH, 1983, The ability of the immunomodulating dipeptide bestatin to activate cytotoxic mononuclear phagocytes. Cancer Research, 43, 4148–4153.
Talmadge JE, Lenz BF, Pennington R, Long C, Philips H, Schneider M and Tribble H, 1986, Immunomodulatory and therapeutic properties of bestatin in mice. Cancer Research, 46, 4505–4510.
Naito S, Von Eschenbach AC and Fidler IJ, 1987, Different growth pattern and biologic behavior of human and renal cell carcinoma implanted into different organs of nude mice. Journal of the National Cancer Institute, 78, 377–385.
Naito S, Walker SM and Fidler IJ, 1989, In vivo selection of human renal cell carcinoma cells with high metastatic potential in nude mice. Clinical and Experimental Metastasis, 7, 381–389.
Nakajima M, Welch DR, Belloni PN and Nicolson GL, 1987, Degradation of basement membrane type IV collagen and lung subendothelial matrix by rat mammary adenocarcinoma cell clone of differing metastatic potentials. Cancer Research, 47, 4869–4876.
Saiki I, Iida J, Murata J, Ogawa R, Nishi N, Sugimura K, Tokura S and Azuma I, 1989, Inhibition of the metastasis of murine malignant melanoma by synthetic polymeric peptides containing core sequence of cell-adhesive molecules. Cancer Research, 49, 3815–3822.
Saiki I, Murata J, Nishi N, Sugimura K and Azuma I, 1989, The inhibition of murine lung metastasis by synthetic polypeptides [poly(arg-gly-asp) and poly (tyr-ile-gly-ser-arg)] with a core sequence of cell adhesion molecures. British Journal of Cancer, 59, 194–197.
Saiki I, Murata J, Iida J, Sakurai T, Nishi M, Matsumoto K and Azuma I, 1989, Antimetastatic effects of synthetic polypeptides containing repeated structures of the cell adhesive Arg-Gly-Asp (RGD) and Tyr-Ile-Gly-Ser-Arg (YIGSR) sequences. British Journal of Cancer, 60, 722–728.
McCarthy JB and Furcht LT, 1984, Laminin and fibronectin promote the haptotactic migration of B16 mouse melanoma cells in vitro. Journal of Cell Biology, 98, 1474–1480.
Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM and McEwan RN, 1987, A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Research, 47, 3239–3245.
Heussen C and Dowdle EB, 1980, Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Analytical Biochemistry, 102, 196–202.
Liotta LA, Abe S, Gehron PR and Martin GR, 1979, Preferential digestion of membrane collagen by an enzyme derived from a metastatic tumor. Proceedings of the National Academy of Science, U. S. A, 76, 2268–2272.
Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM and Shafie S, 1980, Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature, 284, 67–68.
Nicolson GL, 1989, Metastatic tumor cell interactions with endothelium, basement membrane and tissue. Current Opinion in Cell Biology, 1, 1009–1019.
Goldfarb RH, Murano G, Brundage R, Siegal GP, Terranova V, Garbisa S and Liotta LA, 1986, Degradation of glycoprotein and collagenous components of the basement membrane: studies with urokinase-type plasminogen activator, -thrombin and plasmin. Seminar in Thrombosis and Hemostasis, 2, 335–336.
Salo T, Liotta LA, Keski-Oja J, Turpeennieml-Hujamen T and Tryggvason K, 1982, Secretion of basement membrane collagen degrading enzyme and plasminogen activator by transformed cells-role in metastasis. International Journal of Cancer, 30, 669–673.
Liotta LA, Goldfarb RH, Brundage R, Siegal GK, Terranova V and Garbisa S, 1981, Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Research, 41, 4629–4636.
Amoscato AA, Stamkoski RM, Babcock GF and Alexander JW, 1990, Neutral surface amino-peptidase activity of human tumor cell lines. Biochimica et Biophysica Acta, 1041, 317–319.
Ashmun RA and Look AT, 1990, Metalloproteinase activity of CD13/aminopeptidase N on the surface of human myeloid cells. Blood, 75, 462–469.
Morahan PS, Edelson PJ and Gass K, 1980, Changes in macrophages ectoenzymes associated with antitumor activity. Journal of Immunology, 125, 1312–1317.
Wachsmuth ED, 1975, Aminopeptidase as a marker for macrophage differentiation. Experimental Cell Research, 96, 409–412.
Gorvel J-F, Viver I, Naquet P, Brekelmans P, Rigal A and Pierres M, 1990, Characterization of the neutral aminopeptidase activity associated to the mouse thymocyte-activating molecules. Journal of Immunology, 144, 2899–2907.
Poole AR, Tutman KJ, Recklies AD and Stoker TA, 1978, Differences in secretion of the proteinase cathepsin B at the edges of human breast carcinomas and fibroadenomas. Nature, 273, 545–547.
Recklies AD, Mort JS and Poole AR, 1982, Secretion of a thiol proteinase from mouse mammary carcinomas and its characterization. Cancer Research, 42, 1026–1032.
Sloane BF, Honn KV, Sadler JC, Turner WA, Kimpson JJ and Taylor JD, 1982, Cathepsin B activity in B16 melanoma cells: a possible marker for metastasic potential. Cancer Research, 42, 980–986.
Eisenbach L, Segal S and Feldman M, 1985, Proteolytic enzymes in tumor metastasis. II. Collagenase type IV activity in subcellular fractions of cloned tumor cell populations. Journal of the National Cancer Institute, 74, 87–93.
Robertson DM and Williams DC, 1969, In vitro evidence for neutral collagenase activity in an invasive mammalian tumor. Nature, 221, 259–260.
Dano K, Andreasen PA, Grondahl-Hansen J, Kristensen P, Nielsen LS and Skriver L, 1985, Plasminogen activators, tissue degradation and cancer. Advances in Cancer Research, 44, 139–266.
Chop AM, Nakajima M and Nicolson GL, 1989, Metastatic mammary adenocarcinoma cell lines expressed elevated levels of plasma membrane cathepsin H-like and aminopeptidase-like activities. Proceeding of the American Association for Cancer Research, 30, 98.
Suzuki K, Enghild JJ, Morodomi T, Salvesen G and Nagase H, 1990, Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin). Biochemistry, 29, 10261–10270.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yoneda, J., Saiki, I., Fujii, H. et al. Inhibition of tumor invasion and extracellular matrix degradation by ubenimex (bestatin). Clin Exp Metast 10, 49–59 (1992). https://doi.org/10.1007/BF00163576
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00163576