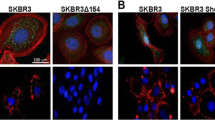
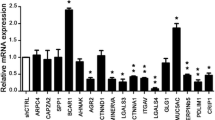
The isoflavinoid genistein is a protein-tyrosine kinase inhibitor which has been identified as a putative cancer prevention agent. Its consumption is associated with a low incidence of clinical metastatic prostate cancer in the face of a sustained high incidence of organ-confined prostate cancer. We therefore undertook studies to examine genistein's effect upon cell adhesion as one possible mechanism by which it could be acting as an antimetastatic agent. A morphogenic analysis revealed that genistein caused cell flattening in a variety of cell lines: PC3-M, PC3, and DU-145 prostate carcinoma cells, as well as MCF-7 breast carcinoma cells. Mechanistic studies focused on the highly metastatic PC3-M cell line, and revealed that cell flattening was accompanied by an increase in cell adhesion. Further investigations demonstrated that focal adhesion kinase (FAK) accumulated in areas of focal cell attachment, and that this accumulation occurred only when cells were actively undergoing genistein-mediated morphologic change. Concurrent formation of a complex between the cell attachment molecule, beta-l-integrin, and FAK was shown to occur, and to correlate with transient activation of FAK activity. Genistein is presented as a novel investigative tool for use in the study of molecular events involved in the process of cell adhesion.
Similar content being viewed by others
References
Pignatelli M and Vessey CJ, 1994, Adhesion molecules: novel molecular tools in tumor pathology. Hum Pathol, 25, 849–56.
Sjaastad MD, Angres B, Lewis RS and Nelson WJ, 1994, Feedback regulation of cell-substratum adhesion by integrin-mediated intracellular Ca2+ signaling. Proc Natl Acad Sci U S A, 91, 8214–8.
Tucker RW, Butterfield CE and Folkman J, 1981, Interaction of serum and cell spreading affects the growth of neoplastic and non-neoplastic fibroblasts. J Supramol Struct Cell Biochem, 15, 29–40.
Wittelsberger SC, Kleene K and Penman S, 1981, Progressive loss of shape-responsive metabolic controls in cells with increasingly transformed phenotype. Cell, 24, 859–66.
Juliano R, 1994, Signal transduction by integrins and its role in the regulation of tumor growth. Cancer Metastasis Rev, 13, 25–30.
Behrens J, 1993, The role of cell adhesion molecules in cancer invasion and metastasis. Breast Cancer Res Treat, 24, 175–84.
Frixen UH, Behrens J, Sachs M, et al. 1991, Ecadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol, 113, 173–85.
Newell KJ, Witty JP, Rodgers WH and Matrisian LM, 1994, Expression and localization of matrix-degrading metalloproteinases during colorectal tumorigenesis. Molec Carcinog, 10, 199–206.
Stracke ML, Murata J, Aznavoorian S and Liotta LA, 1994, The role of the extracellular matrix in tumor cell metastasis. In Vivo, 8, 49–58.
Wang H, Skibber J, Juarez J and Boyd D, 1994, Transcriptional activation of the urokinase receptor gene in invasive colon cancer. Int J Cancer, 58, 650–7.
Behrens J, Mareel MM, Van Roy FM and Birchmeier W, 1989, Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol, 108, 2435–47.
Walter ED, 1941, Genistin (an isoflavone glucoside) and its aglucone, genistein from soybean. J Am Oil Chem Soc, 62, 3273–6.
Eldridge AC, 1982, Determination of isoflavones in soybean flours, protein concentrates, and isolates. J Agric Food Chem, 30, 353–5.
Adlercreutz H, 1990, Western diet and Western diseases: some hormonal and biochemical mechanisms and associations. Scand J Clin Lab Invest, 50, Suppl 201, 3–23.
Mills PK, Beeson WL, Phillips RL and Fraser GE, 1989, Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer, 64, 598–604.
Severson RK, Nomura AM, Grove JS and Stemmermann GN, 1989, A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res, 49, 1857–60.
Akiyama T, Ishida J, Nakagawa S, et al. 1987, Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem, 262, 5592–5.
Bockholt SM and Burridge K, 1993, Cell spreading on extracellular matrix proteins induces tyrosine phosphorylation of tensin. J Biol Chem, 268, 14565–7.
Kornberg L, Earp HS, Parsons JT, Schaller M and Juliano RL, 1992, Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem, 267, 23439–42.
Romer LH, McLean N, Turner CE and Burridge K, 1994, Tyrosine kinase activity, cytoskeletal organization, and motility in human vascular endothelial cells. Molec Biol Cell, 5, 349–61.
Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB and Parsons JT, 1992, ppl25FAK, a structurally distinctive protein-tyrosine kinase associ ated with focal adhesions. Proc Natl Acad Sci USA, 89, 5192–6.
Turner CE, 1994, Paxillin: a cytoskeletal target for tyrosine kinases. Bioessays, 16, 47–52.
Legan PK, Collins JE and Garrod DR, 1992, The molecular biology of desmosomes and hemidesmosomes: what's in a name? Bioessays, 14, 385–93.
Chan PY, Kanner SB, Whitney G and Aruffo A, 1994, A transmembrane-anchored chimeric focal adhesion kinase is constitutively activated and phosphorylated at tyrosine residues identical to pp125FAK. J Biol Chem, 269, 20567–74.
Hatai M, Hashi H, Mogi A, Soga H, Yokota J and Yaoi Y, 1994, Stimulation of tyrosine- and serinephosphorylation of focal adhesion kinase in mouse 3T3 cells by fibronectin and fibroblast growth factor. FEBS Lett, 350, 113–6.
Lipfert L, Haimovich B, Schaller MD, Cobb BS, Parsons JT and Brugge JS, 1992, Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol, 119, 905–12.
Zachary I, Sinnett SJ and Rozengurt E, 1992, Bombesin, vasopressin, and endothelin stimulation of tyrosine phosphorylation in Swiss 3T3 cells. Identification of a novel tyrosine kinase as a major substrate. J Biol Chem, 267, 19031–4.
Kozlowski JM, Isaiah JF, Campbell D, Xu ZL, Kaighn ME and Hart IR, 1984, Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Research, 44, 3522–3529.
Taniguchi S, Iwamura T, Kitamura N, Yamanari H and Setoguchi T, 1994, Heterogeneities of attachment, chemotaxis, and protease production among clones with different metastatic potentials from a human pancreatic cancer cell line. Clin Exp Metastasis, 12, 238–44.
Sartor O, Sameshima JH and Robbins KC, 1991, Differential association of cellular proteins with family protein-tyrosine kinases. J Biol Chem, 266, 6462–6.
Bergan R, Connell Y, Fahmy B and Neckers L, 1993, Electroporation enhances c-myc antisense oligodeoxynucleotide efficacy. Nucleic Acids Res, 21, 3567–73.
Akiyama SK, Larjava H and Yamada KM, 1989, Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. Cell Biol, 109, 863–75.
Burridge K, Turner CE and Romer LH, 1992, Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol, 119, 893–903.
Guan JL and Shalloway D, 1992, Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature, 358, 690–2.
Schweigcrer L, Christeleit K, Fleischmann G, et al. 1992, Identification in human urine of a natural growth inhibitor for cells derived from solid paediatric tumours. Eur J Clin Invest, 22, 260–4.
Adlercreutz H, Markkanen H and Watanabe S, 1993, Plasma concentrations of phyto-oestrogens in Japanese men. The Lancet, 342, 1209–10.
Maroney AC, Lipfert L, Forbes ME, et al. 1995, K-252a induces tyrosine phosphorylation of the focal adhesion kinase and neurite outgrowth in human neuroblastoma SH-SY5Y cells. J Neurochem, 64, 540–9.
Weiner TM, Liu ET, Craven RJ and Cance WG, 1994, Expression of growth factor receptors, the focal adhesion kinase, and other tyrosine kinases in human soft tissue tumors. Ann Surg Oncol, 1, 18–27.
Owens LV, Xu L, Craven RJ, et al. 1995, Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res, 55, 2752–5.
Cance WG and Liu ET, 1995, Protein kinases in human breast cancer. Breast Cancer Res Treat, 35, 105–14.
Schaller MD and Parsons JT, 1994, Focal adhesion kinase and associated proteins. Curr Opin Cell Biol, 6, 705–10.
Hamawy MM, Mergenhagen SE and Siraganian RP, 1993, Tyrosine phosphorylation of pp125FAK by the aggregation of high affinity immunoglobulin E receptors requires cell adherence. J Biol Chem, 268, 6851–4.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bergan, R., Kyle, E., Nguyen, P. et al. Genistein-stimulated adherence of prostate cancer cells is associated with the binding of focal adhesion kinase to beta-l-integrin. Clin Exp Metast 14, 389–398 (1996). https://doi.org/10.1007/BF00123398
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00123398