Abstract
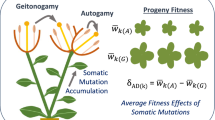
It is hypothesised that somatic mutations are an important source of genetic variance within long-lived plant individuals, and that shoot ontogeny and sexual reproduction are two processes that decrease the mutation load of the shoot population and the offspring. This paper focuses on the way in which sympodial and monopodial shoot branching may influence intra-plant genetic variation and on the role of physiological integration between plant modules for the phenotypic expression of this variation. I also discuss some possible consequences of the interaction of somatic mutations and shoot ontogeny for the study of seedling recruitment and phenotypic plasticity in plant populations.
Similar content being viewed by others
References
Antolin M.F. & Strobeck C. 1985. The population genetics of somatic mutations in plants. Am. Nat. 126: 52–62.
Benner B.L. & Watson M.A. 1989. Developmental ecology of mayapple: seasonal patterns of resource distribution in sexual and vegetative rhizome systems. Funct. Ecol. 3: 539–547.
Bierzychudek P. 1982. Life histories and demography of shade-tolerant temperate forest herbs: a review. New Phytol. 90: 757–776.
Buss L.W. 1987. The evolution of individuality. Princeton University Press, Princeton, USA.
Edwards P.B., Wanjura W.J. & Brown W.V. 1990. Mosaic resistance in plants. Nature 347: 434.
Eriksson O. & Ehrlén J. 1992. Seed and microsite limitation of recruitment in plant populations. Oecologia 91: 360–364.
Geber M.A. 1989. Interplay of morphology and development on size inequality: a Polygonum greenhouse study. Ecol. Monogr. 59: 267–288.
Gill D.E. 1986. Individual plants as genetic mosaics: ecological organisms versus evolutionary individuals. pp. 321–343. In: Crawley M.J. (ed) Plant Ecology. Blackwell Scientific Publications, Oxford, UK.
Hartmannn H.D. & Kester D.E. 1983. Plant propagation. Principles and practices, 4th ed. Prentice-Hall, Inc., Englewood Cliffs, New Jersey, USA.
Hohn B. & Dennis E.S. (eds.) 1985. Genetic flux in plants. Springer Verlag, Wien, Austria.
King L.M. & Schaal B.A. 1990. Genotypic variation within asexual lineages of Taraxacum officinale. Proc. Nat. Acad. Sci. USA 87: 998–1002.
Klekowski E.J.Jr. 1988. Mutation, Developmental Selection, and Plant Evolution. Columbia University Press, New York, USA.
Klekowski E.J.Jr. & Kazarinova-Fukshansky N. 1984a. Shoot apical meristems and mutation: Fixation of selective neutral cell genotypes. Am. J. Bot. 71: 22–27.
Klekowski E.J.Jr. & Kazarinova-Fukshansky N. 1984b. Shoot apical meristems and mutation: selective loss of disadvantageous cell genotypes. Am. J. Bot. 71: 28–34.
Klekowski E.J.Jr., Kazarinova-Fukshansky N. & Fukshansky L. 1989. Patterns of plant ontogeny that may influence genomic stasis. Am. J. Bot. 76: 185–195.
Körner C. & Pelaez Menendez-Riedl S. 1990. The significance of developmental aspects in plant growth analysis. pp. 141–157. In: Lambers H., Cambridge M.L., Konigs H. & Pons T.L. (eds.) Causes and consequences of variation in growth rate and productivity of higher plants. SPB Academic Publishing, The Hague, the Netherlands.
Levins R. & Lewontin R. 1985. The Dialectical Biologist, p 106. Harvard University Press, Cambridge, UK.
Lewontin R.C. 1970. The units of selection. Annu Rev. Ecol. Syst. 1: 1–18.
Lyman J.C. & Ellstrand N.C. 1984. Clonal diversity in Taraxacum officinale (Compositae), an apomict. Heredity 53: 1–10.
Macdonald S.E. & Chinappa C.C. 1989. Population differentiation for phenotypic plasticity in the Stellaria longipes complex. Am. J. Bot. 76: 1627–1637.
Marshall C. 1990. Source-sink relations of interconnected ramets. pp. 23–41. In: vanGroenendael J. & deKroon H. (eds.) Clonal Growth in Plants. Regulation and Function. SPB Academic Publishing, the Hague, the Netherlands.
Mayr E. 1982. The growth of biological thought. Harvard University Press, Cambridge, UK.
Maze J., Banerjee S., El-Kassaby Y.A. & Bohm L.R. 1992. A quantitative genetic analysis of morphological integration in Douglas fir. Int. J. Plant Sci. 153: 333–340.
Novoplansky A. 1991. Developmental responses of Portulaca seedlings to conflicting spectral signals. Oecologia 88: 138–140.
Perttula U. 1941. Untersuchungen über die Generative und Vegetative Vermerung der Blütenpflanzen in der Wald-, Hain-Wiesen und Hainfelsenvegetationen. Ann. Acad. Sci. Fenn. Ser A 58 (1), pp 1–388.
Pedersen, B. 1993. Theoretical studies of life history evolution in modular and clonal organisms. PhD thesis. University of Trondheim, Norway.
Popham R.A. 1951. Principal types of vegetative shoot apex organization in vascular plants. Ohio J. Sci. 51: 249–270.
Salomonson A, Ohlson M. & Ericson L. 1994. Meristem activity and biomass production as response mechanisms in two forest herbs. Oecologia 100: 29–37.
Shaw A.J. 1990. Intraclonal variation in morphology, growth rate and copper tolerance in the moss Funaria hygrometrica. Evolution 44: 441–447.
Slatkin M. 1984. Somatic mutations as an evolutionary force. pp. 19–30. In: Greenwood P.J., Harvey P.H. & Slatkin M. (eds.) Evolution. Essays in honour of John Maynard Smith. Cambridge University Press, Cambridge, UK.
Steeves T.A. & Sussex I.M. 1989. Patterns in plant development. 2nd ed. Cambridge University Press, Cambridge, UK.
Tuomi J. & Vuorisalo T. 1989. Hierarchical selection in modular organisms. Trends Ecol. Evol. 4: 209–213.
Vasseur L., Aarssen L.W. & Bennett T. 1993. Allozymic variation in local apomictic populations of Lemna minor (Lemnaceae). Am. J. Bot. 80: 974–979.
Walbot V. & Cullis C.A. 1985. Rapid genomic change in higher plants. Annu. Rev. Plant. Physiol. 36: 367–396.
Watson M.A. 1984. Developmental constraints: effects on population growth and patterns of resource allocation in a clonal plant. Am. Nat. 123: 411–426.
Watson M.A. 1986. Integrated physiological units in plants. Trends Ecol. Evol. 1: 119–123.
Whitham T.G. & Slobodchikoff C.N. 1981. Evolution by individuals, plant-herbivore interactions, and mosaics of genetic variability: the adaptive significance of somatic mutations in plants. Oecologia 49: 287–292.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Salomonson, A. Interactions between somatic mutations and plant development. Vegetatio 127, 71–75 (1996). https://doi.org/10.1007/BF00054848
Issue Date:
DOI: https://doi.org/10.1007/BF00054848