Abstract
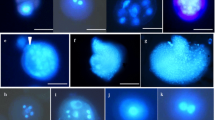
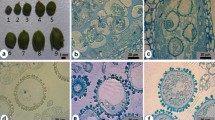
Microspore cryopreservation is a potentially powerful method for long-term storage of germplasm for in vitro embryo production in plant species. In this study, several factors influencing embryo production following the ultra-low temperature (−196 °C in liquid nitrogen) storage of isolated microspores of rapeseed (Brassica napus L.) were investigated. Microspores were prepared in cryogenic vials and subjected to various cooling treatments before immersion in liquid nitrogen for varying periods. Efficiency of microspore cryopreservation was reflected by in vitro embryo production from frozen microspores. Of all the cooling treatments, microspores treated with a cooling rate of 0.25% °C/min and a cooling terminal temperature of −35 °C before immersion in liquid nitrogen produced the highest embryo yields (18% and 40% of unfrozen controls in two genotypes, respectively). Fast thawing in a 35 °C water bath was necessary to recover a high number of embryos from microspore samples being frozen at a higher cooling rate, while thawing speed did not affect samples after freezing at a slower cooling rate. The storage density of cryopreserved microspores affected embryo production. Storage at the normal culture density (8×104 microspores/ml) was less efficient for embryo production than at high densities (4×106 microspores/ml and 1.6×107 microspores/ml), although no significant difference was found between the high densities. Evaluation of plant lines derived from frozen microspores indicated no variation in isozyme pattern and no enhanced cold tolerance of these lines. Isolated microspores of B. napus could be stored for extended period for in vitro embryo production.
Similar content being viewed by others
References
Bajaj YPS (1983) Regeneration of plants from pollenembryos of Arachis, Brassica and Triticum spp. cryopreserved for one year. Curr. Sci. 52: 484–486
Bajaj YPS (1985) Cryopreservation of germplasm of potato (Solanum tubersum L.) and cassava (Manihot esculenta Ctantz): Viability of excised meristems cryopreserved up to four years. Indian J. of Exp. Bio. 23: 285–287
Bajaj YPS (1990) Cryopreservation of germplasm of legumes and oilseed crops. In: Biotechnology in Agriculture and Forestry, Vol 10. Legumes and Oilseed Crops (pp 49–62). Springer Verlags, Berlin Heidelberg, New York, Tokyo
Charne DG (1990) Comparative analyses of microspore-derived and conventional inbred populations of spring oilseed rape (Brassica napus L.). PhD thesis. Department of Crop Science, University of Guelph
Charne DG, Pukacki P, Kott LS & Beversdorf WD (1988) Embryogenesis following cryopreservation in isolated microspores of rapeseed (Brassica napus L.). Plant Cell Rep. 7: 407–409
Chen JL & Beversdorf WD (1990a) A comparison of traditional and haploid-derived populations of oilseed rape (Brassica napus L.) for fatty acid composition of seed oil. Euphytica 51: 59–65
Chen JL & Beversdorf WD (1990b) Fatty acid inheritance in a microspore-derived population of spring rapeseed (Brassica napus L.). Theor. Appl. Genet. 80: 465–469
Chuong PV & Beversdorf WD (1985) High frequency embryogenesis through isolated microspore culture in Brassica napus L. and B. carinata Braun. Plant Sci. 39: 219–226
Coventry J & Kott L (1988) Manual for microspore culture technique for Brassica napus. Crop Science Department, University of Guelph, Guelph, Ontario, Canada
Dussert S, Mauro MC, Deloire A, Hamon S & Engelmann F (1991) Cryopreservation of grape embryogenic cell suspension: 1-Influence of pretreatment, freezing and thawing conditions. Cryo-letter 12: 287–298
Gamborg OL, Miller RA & Ojima K (1968) Nutrient requirements of suspension cultures of soybean root callus. Exp. Cell Res. 50: 151–158
Grout B (1990) Genetic preservation in vitro. In: Nijkamp HJJ, Van der Plas LHW & Van Aartrijk J (Eds) Progress in Plant Cellular and Molecular Biology (pp 13–22). Kluwer Academic Publishers. Dordrecht, Boston, London
Grout B, Morris J & McLellan M (1990) Cryopreservation and the maintenance of cell lines. Tibtech 8: 293–297
Kartha KK (1985a) Cryopreservation of plant cells and organs. Boca Raton Florida, CRC Press, Inc
Kartha KK (1985b) Meristem culture and germplasm preservation. In: Kartha KK (Ed) Cryopreservation of Plant Cells and Organs (pp 115–134). Boca Raton Florida, CRC Press, Inc
Kendall EJ, Qureshi JA, Kartha KK, Leung N, Chevrier N, Caswell K & Chen THH (1990) Regeneration of freezing-tolerant spring wheat (Triticum aestivum L.) plants from cryopreserved callus. Plant Physiol. 94: 1756–1762
Larkin PJ & Scowcroft WR (1981) Somaclonal variation — A novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 60: 197–214
Lichter R (1982) Induction of haploid plants from isolated pollen of Brassica napus. Z. Pflanzenphysiol. 105: 427–434
Mazur P (1965) Causes of injury in frozen and thawed cells. Fed. Proc., 24 (No. 2 Part III) s-175
Polsoni L, Kott LS & Beversdorf WD (1988) Large scale microspore culture techniques for mutation/selection studies in Brassica napus L.. Can. J. Bot. 66: 1681–1685
Reuff I, Seitz U, Ulbrich B & Reinhard E (1988) Cryopreservation of Coleus blumei suspension and callus cultures. J. Plant Physiol. 133: 414–418
Sakai A, Kobayashi S & Oriyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb var brasiliensis Tanaka) by vitrification. Plant Cell Rep. 9: 30–33
Steel RGD & Torrie JH (1980) Principles and procedures of statistics: a biometrical approach. McGraw-Hill, New York
Swanson EB, Coamans MP, Wu SC, Barsby TL & Beversdorf WD (1987) Efficient isolation of microspores and the production of microspore-derived embryos from Brassica napus. Plant Cell Rep. 6: 94–97
Thorpe M, Duke LH & Beversdorf WD (1987) Procedure for the detection of isozymes of rapeseed (Brassica napus and B. campestris) by starch gel electrophoresis. Department of Crop Science, University of Guelph. Technical Bulletin, OAC 887
Uragami A, Sakai A & Nagai M (1990) Cryopreservation of dried axillary buds from plantlets of Asparagus officinalis L. grown in vitro. Plant Cell Rep. 9: 328–331
Uragami A, Sakai A, Nagai M & Takahashi T (1989) Survival of cultured cells and somatic embryos of Asparagus officinalis cryopreserved by vitrification. Plant Cell Rep. 8: 418–421
Yamada T, Sakai A, Matsumura T & Higuchi S (1991) Cryopreservation of apical meristems of white clover (Trifolium repens L.) Plant Science 73: 111–116
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, J.L., Beversdorf, W.D. Cryopreservation of isolated micropores of spring rapeseed (Brassica napus L.) for in vitro embryo production. Plant Cell Tiss Organ Cult 31, 141–149 (1992). https://doi.org/10.1007/BF00037698
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00037698