Abstract
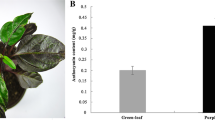
Induction of anthocyanin synthesis occurs during metabolic differentiation in carrot suspension cultured cells grown in medium lacking 2,4-dichlorophenoxyacetic acid (2,4-D), and is closely correlated with embryogenesis. Anthocyanin synthesis may also be induced by light-irradiation under different culture conditions. The phenylalanine ammonia-lyase (PAL) gene (TRN-PAL), which was transiently induced by the transfer effect, was also rapidly induced after light-irradiation. However, TRN-PAL was not involved in anthocyanin synthesis. A second PAL gene, ANT-PAL, was involved in anthocyanin synthesis. ANT-PAL was induced during metabolic differentiation in medium lacking 2,4-D parallel with the induction of chalcone synthase (CHS). PAL genes in the carrot genome are expressed differentially depending on the nature of the environmental stimulus, e.g. transfer effect and light, and other parameters which also affect anthocyanin synthesis.
Similar content being viewed by others
Abbreviations
- CHS:
-
chalcone synthase
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- GUS:
-
β-glucuronidase
- Luc:
-
firefly luciferase
- PAL:
-
phenylalanine ammonia-lyase
- UV:
-
ultraviolet
References
Dixon RA, Dey PM & Lamb CJ (1983) Phytoalexins: Enzymology and molecular biology. Advances in Enzymology and Related Areas of Molecular Biology 55: 1–135
Edwards K, Cramer CL, Bolwell GP, Dixon RA, Schuch W & Lamb CJ (1985) Rapid transient induction of phenylalanine ammonialyase mRNA in elicitor-treated bean cells. Proc. Natl. Acad. Sci. USA 82: 6731–6735
Estabrook EM & Sengupta-Gopalan C (1991) Differential expression of phenylalanine ammonia-lyase and chalcone synthase during soybean nodule development. Plant Cell 3: 299–308
Fujimura T & Komamine A (1975) Effects of various growth regulators on the embryogenesis in a carrot suspension culture. Plant Sci. Lett. 5: 359–364
Gowri G, Paiva NL & Dixon RA (1991) Stress responses in alfalfa (Medicago sativa L.) 12. Sequence analysis of phenylalanine ammonia-lyase (PAL) cDNA clones and appearance of PAL transcripts in elicitor-treated cell cultures and developing plants. Plant Mol. Biol. 17: 415–429
Hahlbrock K & Scheel D (1989) Physiology and molecular biology of phenylpropanoid metabolism. Ann. Rev. Plant Physiol. Plant Mol. Biol. 40: 347–369
Hahlbrock K, Knobloch KH, Kreuzaler F, Potts JRM & Wellmann E (1976) Coordinated induction and subsequent activity changes of two groups of metabolically interrelated enzymes. Light-induced synthesis of flavonoid glycosides in cell suspension cultures of Petroselinum hortense. Eur. J. Biochem. 61: 199–206
Jones DH (1984) Phenylalanine ammonia-lyase: Regulation of its induction, and its role in plant development. Phytochemistry 23: 1349–1359
Kuhn DN, Chappell J, Boudet A & Hahlbrock K (1984) Induction of phenylalanine ammonia-lyase and 4-coumarate:CoA ligase mRNAs in cultured plant cells by UV light or fungal elicitor. Proc. Natl. Acad. Sci. USA 81: 1102–1106
Lawton MA, Dixon RA, Hahlbrock K & Lamb CJ (1983) Elicitor induction of mRNA activity. Rapid effects of elicitor on phenylalanine ammonia-lyase and chalcone synthase mRNA activities in bean cells. Eur. J. Biochem. 130: 131–139
Lois R, Dietrich A, Hahlbrock K & Schulz W (1989) A phenylalanine ammonia-lyase gene from parsley: structure, regulation and identification of elicitor and light responsive cis-acting elements. EMBO J. 8: 1641–1648
Millar AJ, Short SR, Chua NH & Kay SA (1992) A novel circadian phenotype based on firefly luciferase expression in transgenic plants. Plant Cell 4: 1075–1087
Minami E-i, Ozeki Y, Matsuoka M, Koizuka N & Tanaka T (1989) Structure and some characterization of the gene for phenylalanine ammonia-lyase from rice plants. Eur. J. Biochem. 185: 19–25
Ohl S, Hedrick SA, Chory J & Lamb CJ (1990) Functional properties of a phenylalanine ammonia-lyase promoter from Arabidopsis. Plant Cell 2: 837–848
Ozeki Y & Komamine A (1981) Induction of anthocyanin synthesis in relation to embryogenesis in a carrot suspension culture: Correlation of metabolic differentiation with morphological differentiation. Physiol. Plant 53: 570–577
Ozeki Y & Komamine A (1985) Effects of inoculum density, zeatin and sucrose on anthocyanin accumulation in a carrot suspension culture. Plant Cell Tissue Organ Cult. 5: 45–53
Ozeki Y & Komamine A (1986) Effects of growth regulators on the induction of anthocyanin synthesis in a carrot suspension culture. Plant Cell Physiol. 27: 1361–1368
Ozeki Y, Komamine A & Tanaka Y (1990a) Induction and repression of phenylalanine ammonia-lyase and chalcone synthase enzyme proteins and mRNAs in carrot suspension cultures regulated by 2,4-D. Physiol. Plantarum 78: 400–408
Ozeki Y, Matsui K, Sakuta M, Matsuoka M, Ohashi Y, Kano-Murakami Y, Yamamoto N & Tanaka Y (1990b) Differential regulation of phenylalanine ammonia-lyase genes during anthocyanin synthesis and by transfer effect in carrot cell suspension cultures. Physiol. Plantarum 80: 379–387
Sambrook J, Fritsch EF & Maniatis T (1989) Molecular Cloning, a Laboratory Manual, Second Ed. Cold Spring Harbor Laboratory Press, New York
Schröder J, Kreuzaler F, Schafer E & Hahlbrock K (1979) Concomitant induction of phenylalanine ammonia-lyase and flavanone synthase mRNAs in irradiated plant cells. J. Biol. Chem. 254: 57–65
Takeda J (1988) Light-induced synthesis of anthocyanin in carrot cells in suspension. I. The factors affecting anthocyanin production. J. Exp. Bot. 39: 1065–1077
Takeda J (1990) Light-induced synthesis of anthocyanin in carrot cells in suspension. II. Effects of light and 2,4-D on induction and reduction of enzyme activities related to anthocyanin synthesis. J. Exp. Bot. 41: 749–755
Tanaka Y, Matsuoka M, Yamamoto N, Ohashi Y, Kano-Murakami Y & Ozeki Y (1989) Structure and characterization of a cDNA clone for phenylalanine ammonia-lyase from cut-injured roots of sweet potato. Plant Physiol. 90: 1403–1407
Yamada T, Tanaka Y, Sriprasertsak P, Kato H, Hashimoto T, Kawamata S, Ichinose Y, Kato H, Shiraishi T & Oku H (1992) Phenylalanine ammonia-lyase genes from Pisum sativum: Structure, organ-specific expression and regulation by fungal elicitor and suppressor. Plant Cell Physiol. 33: 715–725
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ozeki, Y., Takeda, J. Regulation of phenylalanine ammonia-lyase genes in carrot suspension cultured cells. Plant Cell Tiss Organ Cult 38, 221–225 (1994). https://doi.org/10.1007/BF00033880
Issue Date:
DOI: https://doi.org/10.1007/BF00033880