Abstract
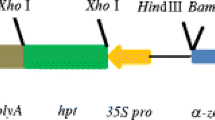
A reproducible and efficient transformation system has been developed for maize that is based on direct DNA uptake into embryogenic protoplasts and regeneration of fertile plants from protoplast-derived transgenic callus tissues. Plasmid DNA, containing the β-glucuronidase (GUS) gene, under the control of the doubled enhancer element (the −208 to −46 bp upstream fragment) from CaMV 35S promoter, linked to the truncated (up to −389 bp from ATG) promoter of wheat, α-amylase gene was introduced into protoplasts from suspension culture of HE/89 genotype. The constructed transformation vectors carried either the neomycin phosphotransferase (NPTII) or phosphinothricin acetyltransferase (PAT) gene as selective marker. The applied DNA uptake protocol has resulted at least in 10–20 resistant calli, or GUS-expressing colonies after treatment of 106 protoplasts. Vital GUS staining of microcalli has made possible the shoot regeneration from the GUS-stained tissues. 80–90% of kanamycin or PPT resistant calli showed GUS activity, and transgenic plants were regenerated from more than 140 clones. Both Southern hybridization and PCR analysis showed the presence of introduced foreign genes in the genomic DNA of the transformants. The chimeric promoter, composed of a tissue specific monocot promoter, and the viral enhancer element specified similar expression pattern in maize plants, as it was determined by the full CaMV 35S promoter in dicot and other monocot plants. The highest GUS specific activity was found in older leaves with progressively less activity in young leaves, stem and root. Histochemical localization of GUS revealed promoter function in leaf epidermis, mesophyll and vascular bundles, in the cortex and vascular cylinder of the root. In roots, the meristematic tip region and vascular tissues stained intensively. Selected transformants were grown up to maturity, and second-generation seedlings with segregation for GUS activity were obtained after outcrossing. The GUS-expressing segregants carried also the NPTII gene as shown by Southern hybridization.
Similar content being viewed by others
References
Battraw MJ, Hall TC: Histochemical analysis of CaMV 35S promoter-β-glucuronidase gene expression in transgenic rice plants. Plant Mol Biol 15: 527–538 (1990).
Beilmann A, Albrecht K, Schultze S, Wanner G, Pfitzner UM: Activation of a truncated PR-1 promoter by endogenous enhancers in transgenic plants. Plant Mol Biol 18: 65–78 (1992).
Benfey PN, Chua NM: The cauliflower mosaic virus 35S promoter: combinatorial regulation of transcription in plants. Science 250: 959–966 (1990).
Benfey PN, Ren L, Chua NH: The CaMV 35S enhancer contains at least two domains which can confer different developmental and tissue-specific expression patterns. EMBO J 8: 2195–2202 (1989).
Benfey PN, Ren L, Chua NH: Tissue-specific expression from CaMV 35S enhancer subdomains in early stages of plant development. The EMBO J 9: 1677–1684 (1989).
Colot V, Robert LS, Kavanagh TA, Bevan MW, Thompson RD: Localization of sequences in wheat endosperm protein genes which confer tissue-specific expression in tobacco. EMBO J 6: 3559–3564 (1987).
Ellis JG, Llewellyn DJ, Dennis ES, Peacock WJ: Maize Adh-1 promoter sequences control anaerobic regulation: addition of upstream promoter elements from constitutive genes is necessary for expression in tobacco. EMBO J 6: 11–16 (1987).
Fang RX, Nagy F, Sivasubramaniam S, Chua NH: Multiple cis regulatory elements for maximal expression of the cauliflower mosaic virus 35S promoter in transgenic plants. Plant Cell 1: 141–150 (1989).
Fromm ME, Taylor LP, Walbot V: Stable transformation of maize after electroporation. Nature 319: 791–793 (1986).
Fromm M, Taylor L, Walbot V: Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci USA 82: 5824–5828 (1985).
Fromm ME, Morrish F, Armstrong C, Williams R, Thomas J, Klein TM: Inheritance and expression of chimeric genes in the progeny of transgenic maize plants. Bio/technology 8: 833–839 (1990).
Golovkin MV, Ábrahám M, Mórocz S, Bottka S, Dudits D: Fertile maize plants from transformed protoplasts and inheritance of introduced foreign gene (submitted).
Gordon-Kamm WJ, Spencer TM, Mangano ML, Adams TR, Danies RJ, Start WG, O'Brien JV, Chambers SA, Adams WR, JrWillets NG, Rice TB, Mackey CJ, Krueger RW, Kausch AP, Lemaux PG: Transformation of maize cells and regeneration of fertile transgenic plants. Plant Cell 2: 603–618 (1990).
Harris RR, DeRobertis GA, Pierce DA, Moynihan MR, Everett NP: Heterogenity of X-gluc staining in transgenic maize callus. In: Abstracts VIIth International Congress on Plant Tissue and Cell Culture, Amsterdam, A5-22, p. 176 (1990).
Huang YW, Dennis ES: Factors influencing stable transformation of maize protoplasts by electroporation. Plant Cell, Tissue Organ Culture 18: 281–296 (1989).
Huttly AK, Baulcombe DC: A wheat α-Amy2 promoter is regulated by gibberellin in transformed oat aleurone protoplasts. EMBO J 8: 1907–1913 (1989).
Jefferson RA, Kavanagh TA, Bevan MW: GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 (1987).
Kay R, Chan A, Daly M, McPherson J: Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science 230: 1299–1302 (1987).
Klein TM, Kornstein L, Sanford JC, Fromm ME: Genetic transformation of maize cells by particle bombardment. Plant Physiol 91: 440–444 (1989).
Krens FA, Molendijk L, Wullems GJ, Schilperoort RA: In vitro transformation of plant protoplasts with Ti-plasmid DNA. Nature 296: 72–74 (1982).
Kyozuka J, Fujimoto H, Izawa T, Shimamoto K: Anaerobic induction and tissue-specific expression of maize Adh1 promoter in transgenic rice plants and their progeny. Mol Gen Genet 228: 40–48 (1991).
Luehrsen KR, Walbot W: Intron enhancement of gene expression and the splicing efficiency of introns in maize cells. Mol Gen Genet 225: 81–93 (1991).
Lyznik LA, Ryan RD, Ritchie SR, Hodges TK: Stable co-transformation of maize protoplasts with gusA and neo genes. Plant Mol Biol 13: 151–161 (1989).
Maas C, Werr W: Mechanism and optimized conditions for PEG mediated DNA transfection into plant protoplasts. Plant Cell Rep 8: 148–151 (1989).
Maniatis T, Fritsch EF, Sambrook J: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY (1982).
Mórocz S, Donn G, Németh J, Dudits D: An improved system to obtain fertile regenerants via maize protoplasts isolated from a highly embryogenic suspension culture. Theor Appl Genet 80: 721–726 (1990).
Nagy F, Boutry M, Hsu MY, Wong M, Chua NH: The 5′-proximal region of the wheat Cab-a gene contains a 268-bp enhancer-like sequence for phytochrome response. EMBO J 6: 2537–2542 (1987).
Oard JH, Paige DF, Simmonds JA, Gradziel TM: Transient gene expression in maize, rice and wheat cells using an airgun apparatus. Plant Physiol 92: 334–339 (1990).
Odell JT, Nagy F, Chua NH: Identification of sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 313: 810–812 (1985).
Planckaert F, Walbot V: Transient gene expression after electroporation of protoplasts derived from embryogenic maize callus. Plant Cell Rep 8: 144–147 (1989).
Reggiardo MI, Arana JL, Orsaria LM, Permingeat HR, Spitteler MA, Vallejos RH: Transient transformation of maize tissues by microparticle bombardment. Plant Sci 75: 237–243 (1991).
Rhodes CA, Lowe KS, Ruby KL: Plant regeneration from protoplasts isolated from embryogenic maize cell cultures. Bio/technology 6: 56–60 (1988).
Somers DA, Birnberg PR, Petersen WL, Brenner ML: The effect of conditioned medium on colony formation from ‘Black Mexican Sweet’ corn protoplasts. Plant Sci 53: 249–256 (1987).
Spencer TM, Gordon-Kamm WJ, Daines RJ, Start WG, Lemaux: Bialaphos selection of stable transformants from maize cell culture. Theor Appl Genet 79: 625–631 (1990).
Stefanov I, Ilubaev S, Fehér A, Margóczi K, Dudits D: Promoter and genotype dependent transient expression of a reporter gene in plant protoplasts. Acta Biol Hung 42: 323–331 (1991).
Terrada R, Shimamoto K: Expression of CaMV 35S-GUS gene in transgenic rice plants. Mol Gen Genet 20: 389–392 (1990).
Vasil V, Clancy M, Ferl RJ, Vasil IK, Hannah LC: Increased gene expression by the first intron maize shrunken-1 locus in grass species. Plant Physiol 91: 1575–1579 (1989).
Zhang W, Wu R: Efficient regeneration of transgenic plants from rice protoplasts and correctly regulated expression of the foreign gene in the plants. Theor Appl Genet 76: 835–840 (1988).
Zhang W, McElroy D, Wu R: Analysis of rice Act1 5′ region activity in transgenic rice plants. Plant Cell 3: 1155–1165 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Omirulleh, S., Ábrahám, M., Golovkin, M. et al. Activity of a chimeric promoter with the doubled CaMV 35S enhancer element in protoplast-derived cells and transgenic plants in maize. Plant Mol Biol 21, 415–428 (1993). https://doi.org/10.1007/BF00028800
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00028800