Abstract
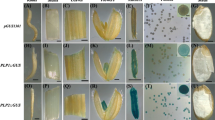
We report here the isolation and characterization of a gene which is specifically expressed during late pollen development in Nicotiana tabacum L. cv. Havana and which exhibits homology to bacterial, fungal and plant polygalacturonases. This gene is ca. 4.3 kb, from the transcription start-site to the 3′ polyadenylation-site sequences. It contains three introns of 620, 706 and 1400 bp and encodes a 1.5 kb message that contains an A-rich 5′-untranslated-leader sequence of 81 bases and a variable-length 3′-untranslated sequence of between 180 and 320 bases. Located within intron 3 is a 414 bp sequence which exhibits 79% homology to a sequence within the endochitinase gene; both sequences share the same internal repeat structure and exhibit features consistent with them being defective transposable elements. The predicted protein sequence coded for by Npg1 shows, in addition to a number of highly conserved cysteines, four conserved domains with the bacterial and fungal polygalacturonase genes. The pollen-specific polygalacturonases as a group can be distinguished from the fruit-ripening polygalacturonases by a number of criteria. It is suggested that these differences reflect the functional differences between plant endo- and exo-polygalacturonases. Npg1 is one of a two-member gene family expressed predominantly in the male gametophyte upon first microspore mitosis. From expression studies of promoter ::GUS transgenes it is clear that the −744 bp to +74/ +85 bp of Npg1 sequence (with respect to the transcription start site) is sufficient to drive the expression of the GUS reporter gene in a manner that reflects the spatial and temporal expression of Npg1 as determined by dot-blot and northern analysis.
Similar content being viewed by others
References
Albani D, Robert LS, Donaldson PA, Altosaar I, Arnison PG, Fabijanski SF: Characterization of a pollen-specific gene family from Brassica napus which is activated during early microspore development. Plant Mol Biol 15: 605–622 (1990).
Albani D, Sardana R, Robert LS, Altosaar I, Arnison PG, Fabijanski SF: A Brassica napus gene family which shows sequence similarity to ascorbate oxidase is expressed in developing pollen. Molecular characterization and analysis of promoter activity in transgenic tobacco plants. Plant J 2: 331–342 (1992).
Allen RL, Lonsdale DM: Sequence analysis of three members of the maize polygalacturonase gene family expressed during pollen development. Plant Mol Biol 20: 343–345 (1992).
Allen RL, Lonsdale DM: Molecular characterization of one of the maize polygalacturonase gene family members, which are expressed during late pollen development. Plant J 3: 261–271 (1993).
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K: Current Protocols in Molecular Biology. John Wiley, New York (1987).
Barakate A, Martin W, Quigley F, Mache R: Characterization of a multigene family encoding an exopolygalacturonase in maize. J Mol Biol 229: 797–801 (1993).
Bird CR, Ray JR, Fletcher JD, Boniwell JM, Bird AS, Teulieres C, Blain I, Bramley PM, Schuch W: Using antisense RNA to study gene function; inhibition of carotenoid biosynthesis in transgenic tomato. Bio/technology 9: 635–639 (1991).
Brown JWS: A catalogue of splice junctions and putative branch point sequences from plant introns. Nucl Acids Res 14: 9549–9559 (1986).
Brown SM, Crouch ML: Characterization of a gene family abundantly expressed in Oenothera organensis pollen that shows sequence similarity to polygalacturonase. Plant Cell 2: 263–274 (1990).
Burnham CR: Discussions in Cytogenetics, 7th ed. University of Minnesota (1984).
Bussink JD, Buxton FP, Visser J: Expression and sequence comparison of the Aspergillus niger and Aspergillus tubigensis genes encoding polgalacturonase II. Curr Genet 19: 467–474 (1991).
Caprari C, Richter A, Bergmann C, Lo Cicero S, Salvi G, Cervone F, De Lorenzo G: Cloning and characterization of a gene encoding the endopolygalacturonase of Fusarium moniliforme. Mycol Res 97: 497–505 (1993).
Devereux J, Haeberli P, Smithies O: A comprehensive set of sequence analysis programs for the VAX. Nucl Acids Res 12: 387–395 (1984).
Dopico B, Lowe AL, Wilson ID, Merodio C, Grierson D: Cloning and characterization of avocado fruit mRNAs and their expression during ripening and low-temperature storage. Plant Mol Biol 21: 437–449 (1993).
Dubald M, Barakate A, Mandaron P, Mache R: The ubiquitous presence of exopolygalacturonase in maize suggests a fundamental cellular function for this enzyme. Plant J, in press (1991).
Fabijanski SF, Albani D, Robert LS, Arnison PG: Characterization of genes expressed during the development of Brassica napus pollen. In Vitro Cell Devel biol 28P: 46–52 (1992).
Frohman MA: In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols: A Guide to Methods and Applications, pp. 28–38. Academic Press, London (1990).
Fukuda Y, Ohme M, Shinshi H: Gene structure and expression of a tobacco endochitinase gene in suspension cultured tobacco cells. Plant Mol Biol 16: 1–10 (1991).
Hamilton AJ, Lycett GW, Grierson D: Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature 346: 284–287 (1990).
Hanson DD, Hamilton DA, Travis JA, Bashe DM, Mascarenhas JP: Characterization of a pollen-specific cDNA clone from Zea mays and its expression. Plant Cell 1: 173–179 (1989).
Henikoff S: Unidirectional digestion with Exonuclease III in DNA sequence analysis. Meth Enzymol 155: 156–165 (1987).
Higgins DG, Sharp PM: CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene 73: 237–244 (1988).
Howland DE, Oliver RP, Davy AJ: A method of extraction of DNA from birch. Plant Mol Biol Rep 9: 340–344 (1991).
Hu MC-T, Davidson N: Mapping transcription start points on cloned genomic DNA with T4 DNA polymerase: a precise and convenient technique. Gene 42: 21–29 (1986).
Jakobsen KS, Breivold E, Hornes E: Purification of mRNA directly from crude plant tissues in 15 minutes using magnetic oligo dT microspheres. Nucl Acids Res 18: 3669 (1990).
Jefferson RA: Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5: 387–405 (1987).
Joshi CP: An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucl Acids Res 15: 6643–6653 (1987).
Joshi CP: Putative polyadenylation signals in nuclear genes of higher plants: a compilation and analysis. Nucl Acids Res 15: 9627–9640 (1987).
Kenton A, Parokonny AS, Gleba YY, Bennett MD: Characterization of the Nicotiana tabacum L. genome by molecular cytogenetics. Mol Gen Genet 240: 159–169 (1993).
Kosak M: Compilation and analysis of sequence upstream from the translational start site in eukaryotic mRNAs. Nucl Acids Res 12: 857–872 (1984).
Lundberg KS, Shoemaker DD, Adams MWW, Short JM, Sorge JA, Mathur EJ: High-fidelity amplification using a thermostable DNA polymerase isolated from Pyrococcus furiosus. Gene 108: 1–6 (1991).
Lütcke HA, Chow KC, Mickel FS, Moss KA, Kern HF, Scheele GA: Selection of AUG initiation codons differs in plants and animals. EMBO J 6: 43–48 (1987).
Manzini G, Barcellona ML, Avitabile M, Quadrifoglio F: Interaction of diamidino-2-phenylindole (DAPI) with natural and synthetic nucleic acids. Nucl Acids Res 11: 8861–8876 (1983).
Mascarenhas JP: The male gametophyte of flowering plants. Plant Cell 1: 657–664 (1989).
Mascarenhas JP: Gene activity during pollen development. Annu Rev Plant Physiol Plant Mol Biol 41: 317–338 (1990).
McElroy D, Blowers AD, Jenes B, Wu R: Construction of expression vectors based on the rice actin 1 (Act1) 5′ region for use in monocot transformation. Mol Gen Genet 231: 150–160 (1991).
Meyerhans A, Vartanian J-P, Wain-Hobson S: DNA recombination during PCR. Nucl Acids Res 18: 1687–1691 (1990).
Murphy G, Kavanagh T: Speeding-up the sequencing of double-stranded DNA. Nucl Acids Res 16: 5198 (1988).
Murphy GJP, Lucas H, Moore G, Flavell RB: Sequence analysis of WIS-2–1A, a retrotransposon-like element from wheat. Plant Mol Biol 20: 991–995 (1992).
Niogret M-F, Dubald M, Mandaron P, Mache R: Characterization of pollen polygalacturonase encoded by several cDNA clones in maize. Plant Mol Biol 17: 1155–1164 (1991).
Popowicz AM, Dash PF: SIGSEQ: a computer program for predicting signal sequence cleavage sites. Comput Appl Biosci 4: 405–406 (1988).
Pressey R: Polygalacturonase in tree pollens. Phytochemistry 30: 1753–1755 (1991).
Pressey R, Reger BJ: Polygalacturonase in pollen from corn and other grasses. Plant Sci 59: 57–62 (1989).
Robert LS, Allard S, Gerster GL, Cass L, Simmonds J: Isolation and characterization of a polygalacturonase gene highly expressed in Brassica napus pollen. Plant Mol Biol 23: 1273–1278 (1993).
Rogers HJ, Allen RL, Hamilton WDO, Lonsdale DM: Pollen specific cDNA clones from Zea mays. Biochim Biophys Acta 1089: 411–413 (1991).
Rogers HJ, Harvey A, Lonsdale DM: Isolation and characterization of a tobacco gene with homology to pectate lyase which is specifically expressed during microsporogenesis. Plant Mol Biol 20: 493–502 (1992).
Rogers HJ, Lonsdale DM: Genetic manipulation of male sterility for the production of hybrid seed. Plant Growth Regul 11: 21–26 (1992).
Ruttkowski E, Khanh NQ, Wientjes F-J, Gottschalk M: Characterization of a polygalacturonase gene of Aspergillus niger RH5344. Mol Microbiol 5: 1353–1361 (1991).
Sachs MM, Dennis ES, Gerlach WL, Peacock WJ: Two alleles of maize alcohol dehydrogenase 1 have 3′ structural and poly(A) addition polymorphisms. Genetics 113: 449–467 (1986).
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA: Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239: 487–491 (1988).
Sanger F, Nicklen S, Coulson AR: DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Shapiro MB, Senapathy P: RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucl Acids Res 15: 7155–7174 (1987).
Sheehy RE, Pearson J, Brady CJ, Hiatt WR: Molecular characterization of tomato fruit polygalacturonase. Mol Gen Genet 208: 30–36 (1987).
Smith CJS, Watson CF, Ray J, Bird CR, Morris PC, Schuch W, Grierson D: Antisense RNA inhibition of polygalacturonase gene expression in transgenic tomatoes. Nature 334: 724–726 (1988).
Staden R: The current status and portability of our sequence handling software. Nucl Acids Res 14: 217–231 (1986).
Tebbutt SJ: Pollen-specific gene expression. Ph.D. thesis, University of East Anglia, UK (1993).
Tebbutt SJ, Lonsdale DM: Identification of a defective transposable element in tobacco. Plant Mol Biol 2: 261–272 (1993).
Thangavelu M, Belostotsky D, Bevan MW, Flavell RB, Rogers HJ, Lonsdale DM: Partial characterization of the Nicotiana tabacum actin gene family: evidence for pollen-specific expression of one of the gene family members. Mol Gen Genet 240: 290–295 (1993).
von Heijne G: A new method for predicting signal sequence cleavage sites. Nucl Acids Res 14: 4683–4690 (1986).
Weterings KAP, Reijen W, van Aarssen R, Kortstee A, Spijkers J, van Herpen M, Schrauwen J, Wullems G: Characterization of a pollen-specific cDNA clene from Nicotiana tabacum expressed during microgametogenesis and germination. Plant Mol Biol 18: 1101–1111 (1992).
Williams JG, Mason PJ: Hybridisation in the analysis of RNA. In: Hames BD, Higgins SJ (eds) Nucleic Acid Hybridisation: A Practical Approach, pp. 139–160. IRL Press, Oxford/Washington DC (1988).
Wing RA, Yamaguchi J, Larabell SK, Ursin VM, McCormick S: Molecular and genetic characterization of two pollen-expressed genes that have sequence similarity to pectate lyases of the plant pathogen Erwinia. Plant Mol Biol 14: 17–28 (1989).
Zuker M, Stiegler P: Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucl Acids Res 9: 133–148 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tebbutt, S.J., Rogers, H.J. & Lonsdale, D.M. Characterization of a tobacco gene encoding a pollen-specific polygalacturonase. Plant Mol Biol 25, 283–297 (1994). https://doi.org/10.1007/BF00023244
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00023244