Abstract
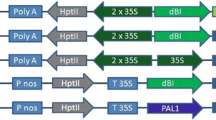
Transcriptional and translational fusions were made between the reading frame coding for β-D-glucuronidase and sequences of either a constitutively expressed rice gene (GOS2) involved in initiation of translation or a light-inducible rice gene (GOS5). The transient expression of the fusions was studied via particle bombardment of seedling tissues of rice, perennial ryegrass and barley. Furthermore, the results of transient and stable expression were compared for cell suspensions of four rice varieties, one barley variety and one perennial ryegrass variety.
The GOS2-gusA fusions were active in all three monocots studied. Best results were obtained for a construct having both a transcriptional and a translational fusion as well as intron and exon sequences (PORCEHyg). The level of GUS activity was in the range of activities as obtained by the 35S CaMV promoter transcriptionally fused to gusA.
The gusA fusion with the light-inducible gene (GOS5) was active in green seedling tissues of all monocots studied. Also a weak expression compared to the GOS2 constructs was found in stably transformed rice callus.
The gusA fusions with the mannopine synthase promoters 1′ and 2′ of the TR-DNA were transiently expressed at lower levels in cell suspensions than PORCEHyg.
For stably transformed rice callus the expression of the GOS2-gusA fusion often decreased during prolonged subculture. This decrease in GUS activity and the various GUS-staining phenotypes of transgenic calli are explained by the presence of different cell types in the suspensions used and in the calli. It is presumed that the nature of the cells and their relative contribution in the calli change drastically upon further subculture.
Similar content being viewed by others
References
Barker, RF, Idler, KB, Thompson, DV, Kemp, JD: Nucleotide sequence of the Agrobacterium tumefaciens octopine Ti plasmid pTi15855. Plant Mol Biol 2: 335–358 (1983).
Battraw, MJ, Hall, TC: Histochemical analysis of CaMV promoter β-glucuronidase gene expression in transgenic rice plants. Plant Mol Biol 15: 527–538 (1990).
Bochardt, A, Hodal, L, Palmgren, G, Mattsson, O, Okkels, FT: DNA methylation is involved in maintenance of an unusual expression pattern of an introduced gene. Plant Physiol 99: 409–414 (1992).
Bradford, NM: A rapid and sensitive method for the detection of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72: 753–760 (1976).
Bradford, MM: A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Bioch 72: 248–254 (1976).
Callis, J, Fromm, M, Walbot, V: Introns increase gene expression in cultured maize cells. Genes Devel 1: 1182–2000 (1987).
Cavener, DR, Ray, SC: Eukaryotic start and stop translation sites. Nucl Acids Res 19: 3185–3192 (1991).
Creemers-Molenaar, J, van derValk, P, Loeffen, JPM, Zaal, MACM: Plant regeneration from suspension cultures and protoplasts of Lolium perenne L. Plant Sci 63: 167–176 (1989).
Datta, SK, Peterhans, A, Datta, K, Potrykus, I: Genetically engineered fertile indica rice recovered from protoplasts. Bio/technology 8: 736–740 (1990).
Davey, MR, Kothari, SL, Zhang, H, Rech, EL, Cocking, EC, Lynch, PT: Transgenic rice: Characterization of protoplast derived plants and their seed progeny. J Exp Bot 42: 1159–1169 (1991).
Dekeyser, R, Claes, B, Marichal, M, VanMontagu, M, Caplan, A: Evaluation of selectable markers for rice transformation. Plant Physiol 90: 217–223 (1989).
Dellaporta, SJ, Wood, J, Hicks, JB: A plant minipreparation: Version II. Plant Mol Biol Rep I 4: 19–21 (1983).
Ellis, JG, Llewellyn, DJ, Walker, JC, Dennis, ES, Peacock, WJ: The ocs element: a 16 bp palindrome essential for activity of the octopine synthetase enhancer. EMBO J 6: 3203–3208 (1987).
Fox, PC, Vasil, V, Vasil, IK, Gurley, WB: Multiple ocs-like elements required for efficient transcription of the mannopine synthase gene of T-DNA in maize protoplasts. Plant Mol Biol 20: 219–233 (1992).
Gallie, DR, Sleat, DE, Watts, JW, Turner, PC, Wilson, TMA: The 5′-leader sequence of tobacco mosaic virus RNA enhances the expression of foreign gene transcripts in vitro and in vivo. Nucl Acids Res 15: 3257–3273 (1987).
Gordon-Kamm, WJ, Spencer, TM: Transformation of maize cells and regeneration of fertile transgenic plants. Plant Cell 2: 603–618 (1990).
Hayashimoto, A, Li, Z, Murai, N: A PEG mediated protoplast transformation system for production of fertile transgenic rice plants. Plant Physiol 93: 857–863 (1990).
Hensgens, LAM, vanOs-Ruygrok, PE: Isolation of RNA and DNA from different rice tissues. Rice Genet Newsl 6: 163–168 (1989).
Hensgens, LAM, Meijer, EGM, vanOs-Ruygrok, PE, Rueb, S, dePater, BS, Schilperoort, RA: Rice transformation, vectors for rice transformation and expression of transferred genes. In: Rice Genetics II, pp. 585–596. International Rice Research Institute, Manila, Philippines (1991).
Hensgens, LAM, Fornerod, MWJ, Rueb, S, Winkler, AA, van derVeen, S, Schilperoort, RA: Translation controls the expression level of a chimaeric reporter gene. Plant Mol Biol 20: 921–938 (1992).
Hensgens LAM, Schilperoort RA: Expression of transferred genes in transgenic rice (tissues) and tobacco. In: Appels R, Raven P, Gustafson JP (eds) Gene Conservation and Exploitation. Plenum, in press.
Hodges TK, Peng J, Lee L, Koetje DS: In: In vitro culture of rice: transformation and regeneration of protoplasts. Proc Stadler Genet Symp 19: 163–183 (1989).
Hoekstra, S, Zijderveld, MH, Louwerse, JD, Heidekamp, F, van derMark, F: Anther and microspore culture of Hordeum vulgare L. cv. Igri. Plant Sci 86: 89–96 (1992).
Jefferson, RA: Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5: 387–405 (1987).
van derKrol, AR, Mur, LA, Beld, M, Mol, JNM, Stuitje, AR: Flavonoid genes in Petunia: addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell 12: 291–299 (1990).
Kyozuka, J, Fujimoto, H, Izawa, T, Shimamoto, K: Anaerobic induction and tissue-specific expression of maize Adh1 promoter in transgenic rice plants and their progeny. Mol Gen Genet 228: 40–48 (1991).
Lazzeri, RA, Brettschneider, R, Lührs, R, Lörz, H: Stable transformation of barley via PEG-induced direct DNA uptake in protoplasts. Theor Appl Genet 18: 437–444 (1991).
Lee, L, Schzoll, RE, Grimes, HD, Hodges, TK: Plant regeneration from indica rice (Oryza sativa L.) protoplasts. Planta 178: 325–333 (1989).
Linn, F, Heidmann, I, Saedler, H, Meyer, P: Epigenetic changes in the expression of the maize A1 gene in Petunia hybrida: Role of numbers of integrated gene copies and state of methylation. Mol Gen Genet 222: 329–336 (1990).
Maas, C, Laufs, J, Grant, S, Korfhage, C, Werr, W: The combination of a novel stimulatory element in the first exon of the maize shrunken-1 gene with the following intron 1 enhances reporter gene expression up to 1000-fold. Plant Mol Biol 16: 199–207 (1991).
Marsh, JL, Erfle, M, Wykes, EJ: The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene 32: 481–485 (1984).
Mascarenhas, D, Mettler, IJ, Pierce, DA, Lowe, HW: Intron-mediated enhancement of heterologous gene expression in maize. Plant Mol Biol 15: 913–920 (1990).
Matsuki, R, Onodera, H, Yamauchi, T, Uchiniya, H: Tissue-specific expression of rolC promoter of the Ri plasmid in transgenic rice plants. Mol Gen Genet 220: 12–16 (1989).
Matzke, MA, Matzke, AJM: Gene interactions and epigenetic variations in transgenic plants. Devel Genet 11: 214–223 (1990).
Matzke, MA, Primig, M, Trnovsky, J, Matzke, AJM: Reversible methylation and inactivation of marker genes in sequentially transformed tobacco plants. EMBO J 8: 643–649 (1989).
McElroy, D, Zhang, W, Wu, R: Isolation of an efficient actin promoter for use in rice transformation. Plant Cell 2: 163–171 (1990).
Meijer, EGM, Schilperoort, RA, Rueb, S, vanOs-Ruygrok, PE, Hensgens, LAM: Transgenic rice cell lines and plants: Expression of transferred chimeric genes. Plant Mol Biol 16: 807–820 (1991).
Meijer, EGM, vanIren, F, Schrijnemakers, E, Hensgens, LAM, vanZijderveld, M, Schilperoort, RA: Retention of the capacity to produce plants from protoplasts in cryopreserved cell lines of rice (Oryza sativa L.). Plant Cell Rep 10: 171–174 (1991).
Müller, AJ, Grafe, R: Isolation and characterization of cell lines of Nicotiana tabacum lacking nitrate reductase. Mol Gen Genet 161: 67–76 (1978).
Murashige, T, Skoog, F: A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 (1962).
Napoli, C, Lemieux, C, Jorgensen, R: Introduction of a chimeric chalcone synthase gene into Petunia hybrida results in reversible co-suppression of homologous genes in trans. Plant Cell 2: 279–289 (1990).
Okkels, JS, Scheller, HV, Jepsen, LB, Moller, BL: A cDNA clone encoding the precursor of a 10.2 kDa photosystem I polypeptide of barley. FEBS Lett 250: 575–579 (1989).
dePater, S, Hensgens, LAM, Schilperoort, RA: Structure and expression of a light-inducible shoot-specific rice gene. Plant Mol Biol 15: 399–406 (1990).
dePater, BS, Schilperoort, RA: Structure and expression of a root-specific rice gene. Plant Mol Biol 18: 161–164 (1992).
dePater, BS, van derMark, F, Rueb, S, Katagari, F, Chua, N-H, Schilperoort, RA, Hensgens, LAM: The promoter of the rice gene GOS2 is active in various different monocot tissues and binds rice nuclear factor ASF-1. Plant J 2: 837–844 (1992).
Peng, J, Lyznik, L, Lee, L, Hodges, TK: Co-Transformation of indica rice protoplasts with gusA and neo genes. Plant Cell Rep 9: 168–172 (1990).
Peralta, EG, Hellmiss, R, Ream, W: Overdrive, a T-DNA transmission enhancer on the A. tumefaciens tumour inducing plasmid. EMBO J 5: 1137–1142 (1986).
Rey P, Diaz C, Schilperoort RA, Hensgens LAM: Cell type-specific expression of three rice genes GOS2, GOS5 and GOS9. Plant Mol Biol in press.
Rueb, S, Hensgens, LAM: Improved histochemical staining for β-D-glucuronidase activity in monocotyledonous plants. Rice Genet Newsl 6: 168–169 (1989).
Sambrook, J, Fritsch, EF, Maniatis, T: Molecular Cloning, A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Scheid, OM, Paszkowski, J, Potrykus, I: Reversible inactivation in Arabidopsis thaliana. Mol Gen Genet 228: 104–112 (1991).
Shimamoto, K, Terada, R, Izawa, T, Fujimoto, H: Fertile transgenic rice plants regenerated from transformed protoplasts. Nature 338: 274–276 (1989).
Tada, Y, Sakamoto, M, Fujimura, T: Efficient gene introduction into rice by electroporation and analysis of transgenic plants: Use of electroporation buffer lacking chloride ions. Theor Appl Genet 80: 475–480 (1990).
Terada, R, Shimamoto, K: Expression of CaMV 35S-GUS gene in transgenic rice plants. Mol Gen Genet 220: 389–392 (1990).
Thompson, JA, Abdulah, R, Cocking, EC: Protoplast culture of rice (Oryza sativa) using media solidified with agarose. Plant Sci 47: 123–133 (1986).
Toriyama, K, Arimoto, Y, Uchimiya, H, Hinata, K: Transgenic rice plants after direct gene transfer into protoplasts. Bio/technology 6: 1072–1074 (1988).
Uchimiya, H, Fushimi, T, Hasimoto, H, Harada, H, Syono, K, Sugawara, Y: Expression of a foreign gene in callus derived from DNA-treated protoplasts of rice (Oryza sativa L.). Mol Gen Genet 204: 204–207 (1986).
Vasil, V, Castillo, AM, Fromm, ME, Vasil, IR: Herbicide resistant fertile transgenic wheat plants obtained by micropojectiles bombardment of regenerable embryogenic callus. Bio/technology 10: 667–674 (1992).
Walbot, V, Gallie, D: Gene Expression in Rice. In: Khush, GS, Toenniessen, GH (eds) Rice Biotechnology, pp. 225–251. Alden Press, Oxford (1991).
Yang, H, Zhang, HM, Davey, MR, Mulligan, BJ, Cocking, EC: Production of kanamycin resistant rice tissues following DNA uptake into protoplasts. Plant Cell Rep 7: 421–425 (1988).
Yoon, H, Donahue, TF: The suil suppressor locus in Saccharomyces cerevisiae encodes a translation factor that functions during tRNA-i-Met recognition of the start codon. Mol Cell Biol 12: 248–260 (1992).
Zhang, HM, Yang, H, Rech, EL, Golds, TJ, Davis, AS, Mulligan, BJ, Cocking, EC, Davis, MR: Transgenic rice plants produced by electroporation-mediated plasmid uptake into protoplasts. Plant Cell Rep 7: 379–384 (1988).
Zhang, W, McElroy, D, Wu, R: Analysis of rice Act1 5′ region activity in transgenic rice plants. Plant Cell 3: 1155–1165 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hensgens, L.A.M., de Bakker, E.P.H.M., van Os-Ruygrok, E.P. et al. Transient and stable expression of gusA fusions with rice genes in rice, barley and perennial ryegrass. Plant Mol Biol 23, 643–669 (1993). https://doi.org/10.1007/BF00021522
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00021522