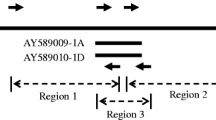
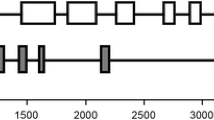
Abstract
A full-length cDNA clone from hexaploid bread wheat, encoding the small subunit of ADP-glucose pyrophosphorylase, has been isolated from an endosperm cDNA library. The cDNA insert has an open reading frame which encodes a protein of 473 amino acids (52.1 kDa). The presence of a chloroplast/amyloplast transit peptide of 22 amino acids is proposed. The deduced amino acid sequence exhibits a high degree of homology with the small subunit ADP-glucose pyrophosphorylase proteins from rice (with 90% of identical amino acids) and potato (with 86% of identical amino acids) and contains conserved sequence elements which are thought to represent the substrate binding and allosteric activator sites. The genes are organised as single-copy loci on chromosomes 7A, 7B and 7D in the wheat genome and are highly expressed during grain development. Homologous transcripts are expressed in leaves and roots.
Similar content being viewed by others
References
Ainsworth CC, Clark J, Balsdon J: Expression, organisation and structure of the genes encoding the waxy protein (granule bound starch synthase) in wheat. Plant Mol Biol 22: 67–82 (1993).
Anderson JM, Hnilo J, Larson R, Okita TW, Morrell M, Preiss J: The encoded primary sequence of a rice seed ADP-glucose pyrophosphorylase subunit and its homology to the bacterial enzyme. J Biol Chem 264: 12238–12242 (1989).
Bae JM, Giroux M, Hannah L: Cloning and characterisation of the brittle-2 gene of maize. Maydica 35: 317–322 (1990).
Baecker PA, Furlong CE, Preiss J: Biosynthesis of bacterial glycogen. Primary structure of Escherichia coli ADP-glucose synthetase as deduced from the nucleotide sequence of the glgC gene. J Biol Chem 258: 5084–5088 (1983).
Bennett MD, Smith JB: Nuclear DNA amounts in angiosperms. Proc R Soc London Ser B 274: 227–274 (1976).
Bhave MR, Lawrence S, Barton C, Hannah LC: Identification and molecular characterisation of shrunken-2 cDNA clones of maize. Plant Cell 2: 581–588 (1990).
Cavaner JR, Ray SC: Eukaryotic start and stop translation sites. Nucl Acids Res 19: 3185–3192 (1991).
Clark JR, Robertson MR, Ainsworth CC: Nucleotide sequence of a wheat (Triticum aestivum L.) cDNA clone encoding the waxy protein. Plant Mol Biol 16: 1099–1101 (1991).
Dickinson DB, Preiss J: Presence of ADP-glucose pyrophosphorylase in shrunken-2 and brittle-2 mutants of maize endosperm. Plant Physiol 46: 1058–1066 (1969).
Feinberg AP, Vogelstein B: A technique for radio-labelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 137: 266–267 (1984).
Haugen TH, Ishaque A, Preiss J: Biosynthesis of bacterial glycogen. Characterisation of the subunit structure of Escherichia coli B glucose-1-phosphate adenylyltransferase. J Biol Chem 251: 7880–7885 (1979).
Keegstra K, Olsen LJ: Chloroplastic precursors and their transport across the envelope membranes. Annu Rev Plant Physiol 40: 471–501 (1989).
Keeling PL, Wood JR, Tyson RH and Bridges IG: Starch biosynthesis in developing wheat grain. Evidence against the direct involvement of triose phosphates in the metabolic pathway. Plant Physiol 87: 311–319 (1988).
Klösgen RB, Gierl A, Schwarz-Sommer Z, Saedler H: Molecular analysis of the waxy locus of maize. Mol Gen Genet 203: 237–244 (1986).
Kozak M: Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell 44: 283–292 (1986).
Lütke HA, Chow KC, Mickel FS, Moss KA, Kern HF, Scheele GA: Selection of AUG initiation codons differs in plants and animals. EMBO J 6: 43–48 (1987).
Maniatis T, Fritsch EF, Sambrook J: In: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1982).
Morell MK, Bloom M, Knowles V, Preiss J: Subunit structure of spinach leaf ADPglucose pyrophosphorylase. Plant Physiol 85: 182–187 (1987).
Muller-Rober BT, Kossmann J, Hannah LC, Willmitzer L, Sonnewald U: One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Mol Gen Genet 224: 136–146 (1990).
Muller-Rober B, Sonnewald U, Willmitzer L: Inhibition of ADP-glucose pyrophosphorylase in transgenic potatoes leads to sugar-storing tubers and influences tuber formation and expression of tuber storage protein genes. EMBO J 11: 1229–1238 (1992).
Nakamura Y, Imamura M: Regulation of ADP-glucose pyrophosphorylase from Chlorella vulgaris. Plant Physiol 85: 601–605 (1985).
Nakata PA, Greene TW, Anderson JM, Smith-White BJ, Okita TW, Preiss J: Comparison of the primary sequences of two potato tuber ADP-glucose pyrophosphorylase subunits. Plant Mol Biol 17: 1089–1093 (1991).
Neuhaus HE, Stitt M: Control analysis of photosynthate partitioning. Impact of reduced activity of ADP-glucose pyrophosphorylase or plastid phosphoglucomutase on the fluxes to starch and sucrose in Arabidopsis thaliana (L.) Heynh. Planta 182: 445–454 (1990).
Olive MR, Ellis RJ, Schuch WW: Isolation and nucleotide sequence of cDNA clones encoding ADP-glucose pyrophosphorylase polypeptides from wheat leaf and endosperm. Plant Mol Biol 12: 525–538 (1989).
Parsons TF, Preiss J: Biosynthesis of bacterial glycogen: Isolation and characterisation of the pyridoxal-P allosteric activator site and the ADP-glucose-protected pyridoxal-P binding site of E. coli B ADP-glucose synthase. J Biol Chem 253: 7638–7645 (1978).
Preiss J: Biosynthesis of starch and its regulation. In: Preiss J (ed) The Biochemistry of Plants, vol 14, pp. 181–254, Academic Press (1988).
Preiss J: Biology and molecular biology of starch synthesis and its regulation. Oxford Surv Plant Mol Cell Biol 7: 59–114 (1991).
Preiss J, Levi C: Starch biosynthesis and degradation. In: Preiss J (ed) The Biochemistry of Plants, vol 3, pp. 371–423 (1980).
Sanger F, Nicklen S, Coulson AR: DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Sanwall GG, Greenberg E, Hardie J, Cameron E, Preiss J: Regulation of starch biosynthesis in plant leaves: activation and inhibition of ADP-glucose pyrophosphorylase. Plant Physiol 43: 417–427 (1968).
Smith-White BJ, Preiss J: Comparison of proteins of ADP-glucose pyrophosphorylase from diverse sources. J Mol Evol 34: 449–464 (1992).
Tsai C, Nelson E: Starch deficient maize mutant lacking Adenosine diphosphate glucose pyrophosphorylase activity. Science 151: 341–343 (1966).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ainsworth, C., Tarvis, M. & Clark, J. Isolation and analysis of a cDNA clone encoding the small subunit of ADP-glucose pyrophosphorylase from wheat. Plant Mol Biol 23, 23–33 (1993). https://doi.org/10.1007/BF00021416
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00021416