Abstract
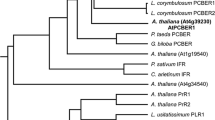
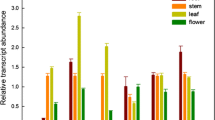
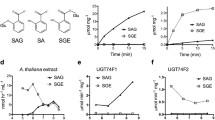
A cDNA clone (RaRO47) encoding a sulfotransferase (ST) has been isolated from Arabidopsis cell suspensions. The deduced polypeptide of 302 amino acids is highly related to plant flavonol sulfotrans-ferases (FSTs), characterized for the first time in Flaveria, and also to STs from animal tissue. The expression of the Arabidopsis ST gene(s) corresponding to RaR047 was examined during different developmental stages. It was found that, at the level of steady-state mRNA, expression of gene(s) encoding this ST was rapidly induced in the aerial parts of young seedlings, and during growth of Arabidopsis cell cultures. No expression could be detected in roots. Treatment of Arabidopsis seedlings with hormonal or stress-related compounds, showed that RaR047 mRNA accumulation was more particularly induced in response to salicylic acid and methyl jasmonate. Furthermore, in the leaves of mature plants or in cell suspensions, accumulation of RaR047 mRNA was observed upon infection with bacterial pathogens. This expression was observed preferentially in response to avirulent pathogens causing an hyper-sensitive reaction, as compared to virulent pathogens, which lead to disease.
Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ: Basic local alignment search tool. J Mol Biol 215: 403–410 (1990).
Ananvoranich S, Varin L, Gulick P, Ibrahim RK: Cloning and regulation of flavonol 3-sulfotransferase in cell-suspension cultures of Flaveria bidentis. Plant Physiol 106: 485–491 (1994).
Ananvoranich S, Gulick P, Ibrahim RK: Flavonol sulfotransferase-like cDNA clone from Flaveria bidentis. Plant Physiol 107: 1019–1020 (1995).
Axelos M, Curie C, Mazzolini L, Bardet C, Lescure B: A protocole for transient gene expression in Arabidopsis thaliana protoplasts isolated from cell suspension cultures. Plant Physiol Biochem 30: 123–128 (1992).
Barron D, Varin L, Ibrahim RK, Harborne JB, Williams CA: Sulphated flavonoids: an update. Phytochemistry 27: 2375–2395 (1988).
Chatterjee B, Majumdar D, Ozbilen O, Murty CVR, Roy AK: Molecular cloning and characterization of cDNA for androgen-repressible rat liver protein, SMP-2. J Biol Chem 262: 822–825 (1987).
Davis KR, Ausubel FM: Characterization of elicitor-induced defense responses in suspension-cultured cells of Arabidopsis. Mol Plant-Microbe Interact 2: 363–368 (1989).
Debener T, Lehnackers H, Arnold M, Dangl JL: Identification and molecular mapping of a single Arabidopsis thaliana locus determining resistance to a pathogenic Pseudomonas syringae isolate. Plant J 1: 289–302 (1991).
Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: version II. Plant Mol Biol Rep 1: 19–21 (1983).
Demyan WF, Song CS, Kim DS, Her S, Gallwitz W, Rao TR, Slomcynska M, Chatterjee B, Roy AK: Estrogen sulfotransferase of the rat liver: complementary DNA cloning and age-and sex-specific regulation of messenger RNA. Mol Endocrinol 6: 589–5978 (1992).
Dixon RA: The phytoalexin response: eliciting, signalling and control of host gene expression. Biol Rev 61: 239–291 (1986).
Dong X, Mindrinos M, Davis KR, Ausubel FM: Induction of Arabidopsis defense genes by virulent and avirulent Pseudomonas syringae strains and by a cloned avirulence gene. Plant Cell 3: 61–72 (1991).
Feinbaum RL, Storz G, Ausubel FM: High intensity and blue light regulated expression of chimeric chalcone synthase genes in transgenic Arabidopsis thaliana plants. Mol Gen Genet 226: 449–456 (1991).
Fenwick GR, Heaney RK, Mullin WJ: Glucosinolates and their breakdown products in food and food-plants. CRC Crit Rev Food Sci Nutr 18: 183–201 (1983).
Gamborg OL: The effects of aminoacids and ammonium on the growth of plant cells in suspension culture. Plant Physiol 45: 372–375 (1970).
Gish W, States DJ: Identification of protein coding regions by database similarity search. Nature Genet 3: 266–272 (1993).
Glendening TM, Poulton JE: Glucosinolate biosynthesis. Sulfation of desulfobenzylglucosinolate by cell free extracts of cress (Lepidium sativum L.) seedlings. Plant Physiol 86: 319–321 (1988).
Godiard L, Ragueh F, Froissard D, Legay JJ, Grosset J, Chartier Y, Meyer Y, Marco Y: Analysis of the synthesis of several pathogenesis-related proteins in tobacco leaves infiltrated with water and with compatible and incompatible isolates of Pseudomonas solanacearum. Mol Plant-Microbe Interact 3: 207–213 (1990).
Gough C, Hemon P, Tronchet M, Lacomme C, Marco Y, Roby D: Developmental and pathogen-induced activation of an msr gene, str 246C, from tobacco involves multiple regulatory elements. Mol Gen Genet 247: 323–337 (1995).
Hahlbrock K, Scheel D: Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Physiol Plant Mol Biol 40: 347–369 (1989).
Hannoufa A, Varin L, Ibrahim RK: Spatial distribution of flavonoid conjugates in relation to glucosyltransferase and sulfotransferase activities in Flaveria bidentis. Plant Physiol 97: 259–263 (1991).
Harborne JB: Flavonoid sulphates: a new class of sulphur compounds in higher plants. Phytochemistry 14: 1147–1155 (1975).
Haughn GW, Davin L, Giblin M, Underhill EW: Biochemical genetics of plant secondary metabolites in Arabidopsis thaliana. The glucosinolates. Plant Physiol 97: 217–226 (1991).
Hemerly AS, Ferreira P, De Almeida Engler J, Van Montagu M, Engler G, Inze D: cdc 2a expression in Arabidopsis is linked with competence for cell division. Plant Cell 5: 1711–1723 (1993).
Jakobek JL, Lindgren PB: Generalized induction of defense responses in bean is not correlated with the induction of the hypersensitive reaction. Plant Cell 5: 49–56 (1993).
Jacobs M, Rubery PH: Naturally occurring auxin transport regulators. Science 241: 346–349 (1988).
Kubasek WL, Shirley BW, McKillop A, Goodman HM, Briggs W, Ausubel FM: Regulation of flavonoid biosynthetic genes in germinating Arabidopsis seedlings. Plant Cell 4: 1229–1236 (1992).
Lamb CJ, Lawton MA, Dron M, Dixon RA: Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell 56: 215–224 (1989).
Lee YC, Park C, Strott CA: Molecular cloning of a chiral-specific 3 alpha-hydroxysteroid sulfotransferase. J Biol Chem 269: 15838–15845 (1994).
Li J, Ou-Lee TM, Raba R, Amundson RG, Last RL: Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 5: 171–179 (1993).
Long SR: Rhizobium-legume nodulation: life together in the underground. Cell 56: 203–214 (1989).
Lummerzheim M, De Oliveira D, Castresana C, Miguens FC, Louzada E, Roby D, Van Montagu M, Timmerman B: Identification of compatible and incompatible interactions between Arabidopsis thaliana and Xanthomonas campestris pv. campestris and characterization of the hypersensitive response. Mol Plant-Microbe Interact 6: 532–544 (1993).
Lutke HA, Chow KC, Mickel FS, Moss KA, Kern HF, Scheele GA: Selection of AUG initiation codon differs in plants and animals. EMBL J 6: 43–48 (1987).
Lynn DG, Chang M: Phenolic signals in cohabitation: implications for plant development. Annu Rev Plant Physiol Plant Mol Biol 41: 497–526 (1990).
Mulder GJ: Sulfation of Drugs and Related Compounds. CRC Press, Boca Raton, FL (1981).
Mulder GJ, Jakoby WB: Sulfation. In: Mulder GJ (ed) Conjugation Reaction in Drug Metabolism, pp. 107–161. Taylor and Francis, New York (1990).
Murashige T, Skoog F: A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–479 (1962).
Nash AR, Glenn WK, Moore SS, Kerr J, Thompson AR, Thompson EO: Oestrogen sulfotransferase: molecular cloning and sequencing of cDNA for the bovine placental enzyme. Aust J Biol Sci 41: 507–516 (1988).
Ogura K, Kajita J, Narihata H, Watabe T, Ozawa S, Nagata K, Yamazoe Y, Kato R: Cloning and sequence analysis of a rat liver cDNA encoding hydroxysteroid sulfotransferase. Biochem Biophys Res Commun 165: 168–174 (1989).
Ohl S, Hedrick SA, Chory J, Lamb CJ: Functional properties of a phenylalanine ammonia-lyase promoter from Arabidopsis. Plant Cell 2: 837–848 (1990).
Otterness DM, Wieben ED, Wood TC, Watson RWG, Madden BJ, McCormick DJ, Weinshilboum RM: Human liver dehydroepiandrosterone sulfotransferase: molecular cloning and expression of cDNA. Mol Pharmacol 41: 865–872 (1992).
Ozawa S, Nagata K, Gong D, Yamazoe Y, Kato R: Nucleotide sequence of a full-length cDNA (PST-1) for arylsulfotransferase from rat liver. Nucl Acid Res 18: 4001–4001 (1990).
Pontier D, Godiard L, Marco Y, Roby D: Hsr203J, a tobacco gene whose activation is rapid, highly localized and specific for incompatible plant/pathogen interactions. Plant J 5: 507–521 (1994).
Raskin I: Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol 43: 439–463 (1992).
Regad F, Bardet C, Tremousaygue D, Moisan A, Lescure B, Axelos M: cDNA cloning and expression of an Arabidopsis GTP-binding protein of the ARF family. FEBS Lett 316: 133–136 (1993).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Shirley BW, Hanley S, Goodman HM: Effects of ionizing radiation on a plant genome: analysis of two Arabidopsis transparent testa mutations. Plant Cell 4: 333–347 (1992).
Truchet G, Roche P, Lerouge P, Vasse J, Camut S, DeBilly F, Prome JC, Denarie J: Sulphated lipo-oligo-saccharide signals of Rhizobium meliloti elicit root nodule organogenesis in alfalfa. Nature 351: 670–673 (1991).
Varin L, Baron D, Ibrahim RK: Enzymatic synthesis of sulphated flavonols in Flaverid. Phytochemistry 26: 135–138 (1987).
Varin L, Ibrahim RK: Partial purification and characterization of three flavonol-specific sulfotransferases from Flaveria chloraefolia. Plant Physiol 90: 977–981 (1989).
Varin L, Ibrahim RK: Partial purification and some properties of flavonol 7-sulfotransferases from Flaveria bidentis. Plant Physiol 95: 1254–1258 (1991).
Varin L, Ibrahim RK: Novel flavonol 3-sulfotransferase. J Biol Chem 267: 1858–1863 (1992).
Varin L, DeLuca V, Ibrahim RK, Brisson N: Molecular characterization of two plant flavonol sulfotransferases. Proc Natl Acad Sci USA 89: 1286–1290 (1992).
Watabe T, Ogura K, Satsukawa M, Okuda H, Hiratsuka A: Molecular cloning and functions of rat liver hydroxysteroid sulfotransferases catalysing covalent binding of carcinogenic polycyclic arylmethanols to DNA. Chem Biol Interact 92: 87–105 (1994).
Weinshilboum R, Otterness DBS: Sulfotransferases enzymes. In: FC Kauffman (ed) Handbook of Experimental Pharmacology: Conjugation-Deconjugation Reactions in Drug Metabolism and Toxicity, vol 112, pp. 45–78. Springer-Verlag, New York (1994).
Wilborn TW, Comer KA, Dooley TP, Reardon IM, Heinrikson RL, Falany CN: Sequence analysis and expression of the cDNA for the phenol-sulfating form of human liver phenol sulfotransferase. Mol Pharmacol 43: 70–77 (1993).
Xu Y, Chang PFL, Narasimhan ML, Raghothama KG, Hasegawa PM, Bressan RA: Plant defense genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell 6: 1077–1085.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lacomme, C., Roby, D. Molecular cloning of a sulfotransferase in Arabidopsis thaliana and regulation during development and in response to infection with pathogenic bacteria. Plant Mol Biol 30, 995–1008 (1996). https://doi.org/10.1007/BF00020810
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00020810