Abstract
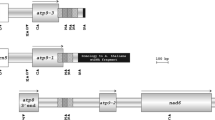
Analyses of mitochondrial transcription and in organello translation were performed with the Brassica tournefortii cytoplasm. This cytoplasm causes alloplasmic male sterility when combined with the nuclear genomes of B. napus and B. juncea. Mitochondrial RNA and protein banding patterns were compared between the fertile wild species B. tournefortii, an alloplasmic male-sterile B. juncea line, an alloplasmic male-sterile B. napus line and an alloplasmic B. napus line with restored fertility. The analyses were carried out to identify differences in gene expression and to investigate whether alterations in gene expression accompanied male sterility. A difference in transcription patterns between the fertile B. tournefortii and the alloplasmic lines was found for the atp6 gene. The atp6 region was investigated further, since a similar alteration in atp6 transcription has been observed in two other Brassica cytoplasms which are associated with cytoplasmic male sterility (CMS). The additional longer atp6 transcript detected in the alloplasmic lines in the present study was found to contain an open reading frame (ORF) located downstream of the atp6 gene. DNA sequencing revealed that the ORF, orf263, could encode a protein with a predicted molecular weight of about 29 kDa. In organello analysis detected two proteins, of 29 and 32 kDa respectively, which were found only in the alloplasmic lines. Furthermore, the 32 kDa protein accompanied male sterility since it was absent in alloplasmic plants restored to fertility. The protein analysis might indicate that orf263 is translated and causes CMS.
Similar content being viewed by others
References
Abad AR, Mehrtens BJ, Mackenzie SA: Specific expression in reproductive tissues and fate of a mitochondrial sterility-associated in cytoplasmic male-sterile bean. Plant Cell 7: 271–285 (1955).
Albaum M, Lührs R, Trautner J, Abel WO: The Tokumasu radish mitochondrial genome contains two complete atp9 reading frames. Plant Mol Biol 29: 179–185 (1995).
Altschul SF, Gish W, Miller W, Myers E, Lipman DJ: Basic local alignment search tool. J Mol Biol 215: 403–410 (1990).
Bernatzky R, Tanksley SD: Genetics of actin-related sequences in tomato. Theor Appl Genet 72: 314–321 (1986).
Bland MM, Matzinger DF, LevingsIII CS: Comparison of the mitochondrial genome of Nicotiana tabacum with its progenitor species. Theor Appl Genet 69: 535–541 (1985).
Bland MM, LevingsIII CS, Matzinger DF: The tobacco mitochodrial ATPase subunit 9 gene is closely linked to an open reading frame for a ribosomal protein. Mol Gen Genet 204: 8–16 (1986).
Bonen L, Boer PH, McIntosh JE, Gray MW: Nucleotide sequence of the wheat mitochondrial gene for subunit I of cytochrome oxidase. Nucl Acids Res 15: 6734 (1987).
Bonhomme S, Budar F, Lancelin D, Small I, Defrance MC, Pelletier G: Sequence and transcript analysis of the Nco2.5 Ogura-specific fragment correlated with cytoplasmic male sterility in Brassica cybrids. Mol Gen Genet 235: 340–348 (1992).
Braun CJ, LevingsIII CS. Nucleotide sequence of the F1-ATPase a subunit gene from maize mitochondrial. Plant Physiol 79: 571–577 (1985).
Braun CJ, Brown G G, LevingsIII CS: Cytoplasmic male sterility. In: Hermann RG (ed) Cell Organelles. Plant Gene Research, pp. 219–245. Springler-Verlag, New York (1992).
Chase CD, Ortega VM: Organization of ATPA coding and 3′ flanking sequences associated with cytoplasmic male sterility in Phaseolus vulgaris L. Curr Genet 22: 147–153 (1992).
Dale RMK, Mendu N, Ginsburg H, Kridl JC: Sequence analysis of maize mitochondrial 26S rRNA gene and flanking regions. Plasmid 11: 141–150 (1984).
Day DA, Neuburger A, Douce R: Biochemical characterization of chlorophyll-free mitochondria from pea leaves. Aust J Plant Physiol 12: 219–228 (1985).
Dawson AJ, Jones VP, Leaver CJ: The apocytochrome b gene in maize mitochondria does not contain introns and is by a potential ribosome binding site. EMBO J 3: 2107–2113 (1984).
Dewey RE, LevingsIII CS: Nucleotide sequence of ATPase δ gene of maize mitochondria. Plant Physiol 79: 914–919 (1985).
Dewey RE, LevingsIII CS, Timothy DH: Novel recombinations in the maize mitochondrial genome produce a unique transcriptional unit in the Texas male-sterile cytoplasm. Cell 44: 439–449 (1986).
Dewey RE, Timothy DH, LevingsIII CS: A mitochondrial protein associated with cytoplasmic male sterility in the T cytoplasm of maize. Proc Natl Acad Sci USA 84: 5374–5378 (1987).
Fox TD, Leaver CJ: The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codons. Cell 26: 315–323 (1981).
Grelon M, Budar F, Bonhomme S, Pelletier G: Ogura cytoplasmic male-sterility (CMS)-associated orf1 38 is translated into a mitochondrial membrane polypeptide in male-sterile Brassica cybrids. Mol Gen Genet 243: 540–547 (1994).
Gwynn B, Dewey RE, Sederoff RR, Timothy DH, LevingsIII CS: Sequence of the 18S-5S ribosomal gene region and the cytochrome oxidase II gene from mtDNA of Zea diploperennis. Theor Appl Genet 74: 781–788 (1987).
Handa H, Nakajima K: Different organization and altered transcription of the mitochondrial atp6 gene in the male-sterile cytoplasm of rapeseed (Brassica napus L.). Curr Genet 21: 153–159 (1992).
Hanson MR: Plant mitochondrial mutations and male sterility. Annu Rev Genet 25: 461–486 (1991).
Hernould M, Suharsono S, Litvak S, Arava A. Mouras A: Male-sterility induction in transgenic tobacco plants with an unedited atp9 mitochondrial gene from wheat. Proc Natl Acad Sci USA 90: 2370–2374 (1993).
Hiesel R, Schobel W, Schuster W, Brennicke A: The cytochrome oxidase subunit I and subunit III genes in Oenothera mitochondria are transcribed from identical promoter sequences. EMBO J 6: 29–34 (1987).
Håkansson G, van derMark, Bonnett HT, Glimelius K: Variant mitochondrial protein and DNA patterns associated with cytoplasmic male-sterile lines of Nicotiana. Theor Appl Genet 76: 431–437 (1988).
Håkansson G, Glimelius K: Extensive nuclear influence on mitochondrial transcription and genome structure in malefertile and male-sterile alloplasmic Nicotiana materials. Mol Gen Genet 229: 380–388 (1991).
Iwabuchi M, Kyozuka J, Shimamoto K: Processing followed by complete editing of an altered mitochondrial atp6 RNA restores fertility of cytoplasmic male-sterile rice. EMBO J 12: 1437–1446 (1993).
Johns C, Meiqing L, Lyznik A, Mackenzie S: A mitochondrial DNA sequence is associated with abnormal pollen development in cytoplasmic male-sterile bean plants. Plant Cell 4: 435–449 (1992).
Knoop V, Schuster W, Wissinger B, Brennicke A: Transsplicing integrates an exon of 22 nuclotides into the nad5 mRNA in higher plant mitochondria. EMBO J 10: 3483–3493 (1991).
Krishnasamy S, Makaroff CA: Characterization of the radish mitochondrial orfB locus: possible relationship with male sterility in Ogura radish. Curr Genet 24: 156–163 (1993).
Krishnasamy S, Grant RA, Makaroff CA: Subunit 6 of the FO-ATP synthase complex from cytoplasmic male-sterile radish: RNA editing and NH2-terminal protein sequencing. Plant Mol Biol 24: 129–141 (1994).
Krishnasamy S, Makaroff CA: Organ-specific reduction in the abundance of a mitochondrial protein accompanies fertility restoration in cytoplasmic male-sterile radish. Plant Mol Biol 26: 935–946 (1994).
Köhler RH, Horn R, Lössl A, Zetsche K: Cytoplasmic male sterility in sunflower is correlated with the co-transcription of a new open reading frame with the atpA gene. Mol Gen Genet 227: 369–376 (1991).
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriphage T4. Nature 227: 680–685 (1970).
Landgren M, Glimelius K: Analysis of chloroplast and mitochondrial segregation in three different combinations of somatic hybrids produced within Brassicaceae. Theor Appl Genet 80: 776–784 (1990).
Laver HK, Reynolds SJ, Monéger F, Leaver CJ: Mitochondrial genome organization and gene expression associated with cytoplasmic male sterility in sunflower (Helianthus annuus). Plant J 1: 185–193 (1991).
L'Homme Y, Brown GG: Organizational differences between cytoplasmic male-sterile and male fertile Brassica mitochondrial genomes are confined to a single transposed locus. Nucl Acids Res 21: 1903–1909 (1993).
Liu JH, Landgren M, Glimelius K: Transfer of the B. tournefortii cytoplasm to B. napus for the production of cytoplasmic male sterile B. napus. Physiol Plant 96: 123–129 (1996).
Logemann J, Schell J, Willmitzer L: Improved method for the isolation of RNA from plant tissues. Anal Biochem 163: 16–20 (1987).
Lonsdale DM: The plant mitochondrial genome. In: Marcus J (ed) The Biochemistry of Plants. vol 15, pp. 229–295. Academic Press, New York (1989).
Makaroff CA, Apel IJ, Palmet JD: The atp6 coding region has been disrupted and a novel reading frame generated in the mitochondrial genome of cytoplasmic male-sterile radish. J Biol Chem 264: 11606–11713 (1989).
Makaroff CA, Apel IJ, Palmer JD: The role of coxI-associated repeated sequences in plant mitochondrial DNA rearrangements and radish cytoplasmic male sterility. Curr Genet 19: 183–190 (1991).
Mathias R: Transfer of cytoplasmic male sterility from brown mustard (Brassica juncea (L.) Coss.) into rapeseed (Brassica napus L.). Z Pflanzenzücht 95: 371–374 (1985).
Monéger F, Smart CJ, Leaver CJ: Nuclear restoration of cytoplasmic male sterility in sunflower is associated with the tissue-specific regulation of a novel mitochondrial gene. EMBO J 13: 8–17 (1994).
Nivison HT, Hanson MR: Identification of a mitochondrial protein associated with cytoplasmic male sterility in petunia. Plant Cell 1: 1121–1130 (1989).
Palmer JD, Shields CR: Tripartite structure of the Brassica campestris mitochondrial genome. Nature 307: 437–440 (1984).
Pradhan AK, Mukhopadhyay A, Pental D: Identification of the putative cytoplasmic donor of a CMS system in Brassica juncea. Plant Breed 106: 204–208 (1991).
Rawat DS, Anand IJ: Male sterility in Indian mustard. Indian J Genet Plant Breed 39: 412–414 (1979).
Sambrook E, Fritsch F, Maniatis T: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Sanger F, Nicklen S, Coulson AR: DNA sequencing with chainterminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Schuster W: Ribosomal protein gene rpl5 is cotranscribed with nad3 gene in Oenothera mitochondria. Mol Gen Genet 240: 445–449 (1993).
Singh M, Brown GG: Suppression of cytoplasmic male sterility by nuclear genes alters expression of a novel mitochondrial gene region. Plant Cell 3: 1349–1362 (1991).
Sodhi YS, Pradhan AK, Verma JK, Arumugam N, Mukhopadhyay A, Pental D: Identification and inheritance of fertility restorer genes for ‘tour’ CMS in rapeseed (Brassica napus L.). Plant Breed 12: 223–227 (1994).
Stamper SE, Dewey RE, Bland MM, LevingsIII CS: Characterization of the gene urf13-T and an unidentified reading frame, ORF 25, in maize and tobacco mitochondria. Curr Genet 12: 457–463 (1987).
Szasz A, Landgren M, Fahleson J, Glimelius K: Characterization and transfer of the male-sterile Anand cytoplasm from Brassica juncea to Brassica napus via protoplast fusion. Physiol Plant 82: A29 (1991).
Verwoerd TC, Dekker BM, Hoekema A: A small-scale procedure for the rapid isolation of plant RNAs. Nucl Acids Res 17: 2362 (1989).
Young EG, Hanson MR: A fused mitochondrial gene associated with cytoplasmic male sterility is developmentally regulated. Cell 50: 41–49 (1987).
Zetsche K, Horn R: Molecular analysis of cytoplasmic male sterility in sunflower (Helianthus annuus). In: Brennicke A, Kück U (eds) Plant Mitochondria with Emphasis on RNA Editing and Cytoplasmic Male Sterility, pp. 411–422. VCH, Weinheim (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Landgren, M., Zetterstrand, M., Sundberg, E. et al. Alloplasmic male-sterile Brassica lines containing B. tournefortii mitochondria express an ORF 3′ of the atp6 gene and a 32 kDa protein. Plant Mol Biol 32, 879–890 (1996). https://doi.org/10.1007/BF00020485
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00020485