Abstract
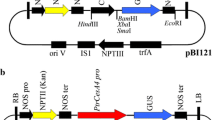
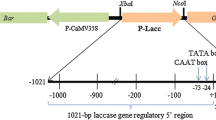
Cinnamyl alcohol dehydrogenase (CAD) which catalyses the synthesis of the cinnamyl alcohols, the immediate precursors of lignins, from the corresponding cinnamaldehydes is considered to be a highly specific marker for lignification We have isolated and characterized a CAD genomic clone from eucalyptus, a woody species of economic importance. The full-length promoter (EuCAD, 2.5 kb) and a series of 5′ deletions were fused to the β-glucuronidase (GUS) reporter gene. These constructs were tested in a homologous transient expression system of eucalyptus protoplasts which enabled the identification of several regions involved in transcriptional control. In order to study the spatial and developmental regulation of the CAD gene, the chimeric gene fusion (EuCAD-GUS) was then transferred via Agrobacterium tumefaciens-mediated transformation into poplar, an easily transformable woody angiosperm. Quantitative fluorometric assays conducted on eight independent in vitro transformants showed that GUS activity was highest in roots followed thereafter by stems and leaves. Histochemical staining for GUS activity on both in vitro primary transformants and more mature greenhouse-grown plants indicated a specific expression in the vascular tissues of stems, roots, petioles and leaves. At the onset of xylem differentiation, GUS activity was detected in parenchyma cells differentiating between the xylem-conducting elements. After secondary growth has occurred, GUS activity was localized in xylem ray cells and parenchyma cells surrounding the lignified phloem and sclerenchyma fibers. This first characterization of a woody angiosperm CAD promoter provides functional evidence for the role of CAD in lignification and suggests that parenchyma cells expressing CAD may provide lignin precursors to the adjacent lignified elements (vessels and fibres).
Similar content being viewed by others
References
An C, Ichinose Y, Yamada T, Tanaka Y, Shiraishi T, Oku H: Organization of the genes encoding chalcone synthase in Pisum sativum. Plant Mol Biol 21: 789–803 (1993).
An G, Edbert PR, Mitra A, Ha SB: Binary vectors. In: Gelvin SB, Shilperoort RA, Verma DPS (eds) Plant Molecular Biology Manual, pp. 1–9. Kluwer Academic Publishers, Dordrecht (1988).
Axelos M, Bardet C, Liboz T, Le VanThai A, Curie C, Lescure B: The gene family encoding the Arabidopsis thaliana translation elongation factor EF-1α: molecular cloning, characterization and expression. Mol Gen Genet 219: 106–112 (1989).
Bevan M: Binary Agrobacterium vectors for plant transformation. Nucl Acids Res 12: 22 (1984).
Bevan M, Shufflebottom D, Edwards K, Jefferson R, Schuch W: Tissue and cell-specific activity of a phenylalanine ammonia-lyase promoter in transgenic plants. EMBO J 8: 1899–1906 (1989).
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 (1976).
Cramer CL, Edwards K, Dron M, Liang X, Dildine SL, Bolwell GP, Dixon RA, Lamb J, Schuch W: Phenylalanine ammonia-lyase gene organization and structure. Plant Mol Biol 12: 367–383 (1989).
DaCosta e Silva O, Klein L, Schmelzer E, Trezzini GF, Hahlbrock K: BPF-1, a pathogen-induced DNA binding protein involved in the plant defense response. Plant J 4: 125–135 (1993).
Darvill AG, Albersheim P: Phytoalexins and their elicitors: a defense against microbial infection in plants. Annu Rev Plant Physiol 35: 243–275 (1984).
Dean C, Tamaki S, Dunsmuir P, Favreau M, Katayama C, Dooner H, Bedbrook J: mRNA transcripts of several plant genes are polyadenylated at multiple sites in vivo. Nucl Acids Res 14: 2229–2240 (1986).
DeBlock M: Factors influencing the tissue culture and the Agrobacterium tumefaciens-mediated transformation of hybrid aspen and poplar clones. Plant Physiol 93: 1110–1116 (1990).
Dellaporta SL, Wood J, Hicks JB: A plant DNA minipreparation: version II. Plant Mol Biol Rep 1: 19–21 (1983).
Douglas C, Hoffman H, Schulz W, Hahlbrock K: Structure and elicitor or UV-light stimulated expression of two 4-coumarate: CoA ligase genes in parsley. EMBO J 6: 1189–1195 (1987).
Dron M, Clouse SD, Dixon RA, Lawton MA, Lamb CJ: Glutathione and fungal elicitor regulation of a plant defense gene promoter in electroporated protoplasts. Proc Natl Acad Sci USA 85: 6738–6742 (1988).
Esau K: Anatomy of Seed Plants, 2nd ed. J. Wiley, New York (1977).
Feuillet C, Boudet AM, Grima-Pettenati J: Nucleotide sequence of a cDNA encoding cinnamyl alcohol dehydrogenase from eucalyptus. Plant Physiol 103: 1447 (1994).
Freudenberg K: Lignin: its constitution and formation from p-hydroxycinnamyl alcohols. Science 148: 595–600 (1965).
Galliano H, Heller W, Sandermann H: Ozone induction and purification of spruce cinnamyl alcohol dehydrogenase. Phytochemistry 32: 557–563 (1993).
Goffner D, Joffroy I, Grima-Pettenati J, Halpin C, Knight M, Schuch W, Boudet AM: Purification and characterization of isoforms of cinnamyl alcohol dehydrogenase (CAD) from eucalyptus xylem. Planta 188: 48–53 (1992).
Goring DAI: Polymer properties of lignin and lignin derivatives. In: Sarkanen KV, Ludwig GH (eds) Lignins: Occurrence, Formation, Structure and Reactions, pp. 695–768. Wiley Interscience, New York (1971).
Grima-Pettenati J, Feuillet C, Goffner D, Borderies G, Boudet AM: Molecular cloning and expression of a Eucalyptus gunnii cDNA clone encoding cinnamyl alcohol dehydrogenase. Plant Mol Biol 21: 1085–1095 (1993).
Haigler C: Metabolite storage and vesicle transport associated with lignification. DOE Lignin Workshop: Research Needs in Lignin Biosynthesis and Biodegradation, Asilomar (USA), 24–27 May 1994, pp. 7–11 (1994).
Hauffe KD, Paszkowski U, Schulze-Lefert P, Halbrock K, Dangl JL, Douglas CJ: A parsley 4CL-1 promoter fragment specifies complex expression patterns in transgenic tobacco. Plant Cell 3: 435–443 (1991).
Hibino T, Shibata D, Chen J, Higushi T: Cinnamyl alcohol dehydrogenase from Aralia cordata: cloning of the cDNA and expression of the gene in lignified tissues. Plant Cell Physiol 34: 659–665 (1993).
Hibino H, Chen J, Shibata D, Higushi T: Nucleotide sequence of a Eucalyptus botryoides gene encoding cinnamyl alcohol dehydrogenase. Plant Physiol 104: 305–306 (1994).
Hollick JB, Gordon MP: A poplar tree proteinase inhibitor-like gene promoter is responsive to wounding in transgenic tobacco. Plant Mol Biol 22: 561–572 (1993).
Jefferson RA, Kavanagh TA, Bevan MW: GUS fusions: β-glucuronidase as a sensitive and versatile marker in higher plants. EMBO J 6: 3901–3907 (1987).
Joshi CP: An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucl Acids Res 15: 6643–6653 (1987).
Klopfenstein NB, Shi NQ, Kernan A, McNabb HS, Hall RB, Hart ER, Thornburg RW: Transgenic Populus expresses a wound-inducible potato proteinase inhibitor II-CAT gene fusion. Can J Forest Res 21: 1321–1328 (1991).
Knight ME, Halpin C, Schuch W: Identification and characterisation of cDNA clones encoding cinnamyl alcohol dehydrogenase from tobacco. Plant Mol Biol 19: 793–801 (1992).
Koncz C, Shell J: The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204: 383–396 (1986).
Lawton MA, Dean SM, Dron M, Kooter JM, Kragh KM, Harrison MJ, Yu L, Tanguay L, Dixon RA, Lamb CJ: Silencer region of a chalcone synthase promoter contains multiple binding site for a factor, SBF-1, closely related to GT-1. Plant Mol Biol 16: 235–249 (1991).
Leinhos V, Savidge RA: Isolation of protoplasts from developping xylem of Pinus banksiana and Pinus strobus. Can J Forest Res 23: 343–348 (1993).
Leple JC, Brasileiro ACM, Michel MF, Delmotte F, Jouanin L: Transgenic poplars: expression of chimeric genes using different constructs. Plant Cell Rep 11: 137–141 (1992).
Liang X, Dron M, Schmid J, Dixon RA, Lamb CJ: Developmental and environmental regulation of a phenylalanine ammonia-lyase-β-glucuronidase gene fusion in transgenic tobacco plants. Proc Natl Acad Sci USA 86: 9284–9288 (1989).
Lois R, Dietrich A, Hahlbrock K, Schulz W: A phenylalanine ammonia-lyase gene from parsley: structure, regulation and identification of elicitor and light responsive cis-acting elements, EMBO J 8: 1641–1648 (1989).
MacLauchlan J, Gaffney D, Whitton JL, Clements JB: The consensus YGTGTTYY located downstream from the AATAAA signal is required for efficient formation of mRNA 3′ termini. Nucl Acids Res 13: 1347–1368 (1985).
Minami E, Ozeki Y, Matsuoka M, Koizuka N, Tanaka Y: Structure and some characterization of the gene for phenylalanine ammonia-lyase from rice plants. Eur J Biochem 185: 19–25 (1989).
Ohl S, Hedrick SA, Chory J, Lamb CJ: Functional properties of a phenylalanine ammonia-lyase promoter from Arabidopsis. Plant Cell 2: 837–848 (1990).
O'Malley DM, Porter S, Sederoff RR: Purification, characterization and cloning of cinnamyl alcohol dehydrogenase in loblolly pine (Pinus taeda L.). Plant Physiol 98: 1364–1371 (1992).
Parsons TJ, Sinkar VP, Stettler RF, Nester EW, Gordon MP: Transformation of poplar by Agrobacterium tumefaciens. Bio/technology 4: 533–536 (1986).
Pickett-Heaps JD: Xylem wall deposition: radioautographic investigations using lignin precursors. Protoplasma 65: 181–205 (1968).
Proudfoot NJ, Brownlee GG: 3′ non coding region sequences in eucaryotic mRNA. Nature 263: 211–214 (1976).
Roberts AW, Koonce LT, Haigler CH: A simplified medium for in vitro tracheary element differentiation in mesophyll suspension cultures of Zinnia elegans L. Plant Cell, Tissue and Organ Culture 28: 27–35 (1992).
Ryser U, Keller B: Ultrastructural localization of a bean glycine-rich protein in unlignified primary walls of protoxylem cells. Plant Cell 4: 773–783 (1992).
Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning; A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Sanger F, Nicklen S, Coulson AR: DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Sederoff R, Campbell M, O'Malley D, Whetten R: Genetic regulation of lignin biosynthesis and the potential modification of wood by genetic engineering. In: Ellis B et al. (eds) Genetic Engineering of Plant Secondary Metabolism, pp. 313–355. Plenum Press, New York (1994).
Shufflebottom D, Edwards K, Schuch W, Bevan M: Transcription of two members of a gene encoding phenylalanine ammonia-lyase leads to remarkably different cell specificities and induction patterns. Plant J 3: 835–845 (1993).
Smith CG, Rodgers MW, Zimmerlin A, Fernandino D, Bolwell GP: Tissue and subcellular immunolocalisation of enzymes of lignin synthesis in differentiating and wounded hypocotyl tissue of french bean (Phaseolus vulgaris L.). Planta 192: 155–164 (1994).
Sommer H, Saedler H: Structure of the chalcone synthase gene of Antirrhinum majus. Mol Gen Genet 202: 429–434 (1986).
Subramaniam R, Reinold S, Molitor EK, Douglas CJ: Structure, inheritance and expression of hybrid poplar (Populus trichocarpa × Populus deltoides) phenylalanine ammonia-lyase genes. Plant Physiol 102: 71–83 (1993).
Teulieres C, Feuillet C, Boudet AM: Differential characteristics of cell suspension culture initiated from Eucalyptus gunnii clones differing by their frost tolerance. Plant Cell Rep 8: 407–410 (1989).
Teulieres C, Grima-Pettenati J, Curie C, Teissie J, Boudet AM: Transient foreign gene expression in polyethylene/glycol treated or electropulsated Eucalyptus gunnii protoplasts. Plant Cell Tissue Organ Culture 25: 125–132 (1991).
Van Doorsselaere J, Van Der Mijnsbrugge K, Baucher M, Leple JC, Rhode A, Van Montagu M, Inze D: Genetic engineering in forest trees. Agro-food-Industry Hitech, pp. 15–19 (1993).
Vorst O, Kock P, Lever A, Weterings B, Weisbeek P, Smeekens S: The promoter of Arabidopsis thaliana plastocyanin gene contains a far upstream enhancer-like element involved in chloroplast-dependent expression. Plant J 4: 933–945 (1993).
Walter MH: Regulation of lignification in defense. In: Boller T, Meins F (eds) Plant Gene Research: Genes Involved in Plant Defense, pp. 327–352. Springer, Wien (1992).
Walter MH, Schaaf J, Hess D: Gene activation in lignin biosynthesis: pattern of promoter activity of a tobacco cinnamyl alcohol dehydrogenase gene. Acta Hort (in press) (1994).
Wardrop AB: Lignins: Occurrence and formation in plants. In: Sarkanen KV, Ludwig GH (eds) Lignins: Occurrence, Formation, Structure and Reactions, pp. 19–41. Wiley Interscience, New York (1971).
Wilde HD, Meagher RB, Merkle SA: Expression of foreign genes in transgenic yellow poplar plants. Plant Physiol 98: 114–120 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Feuillet, C., Lauvergeat, V., Deswarte, C. et al. Tissue- and cell-specific expression of a cinnamyl alcohol dehydrogenase promoter in transgenic poplar plants. Plant Mol Biol 27, 651–667 (1995). https://doi.org/10.1007/BF00020220
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00020220