Abstract
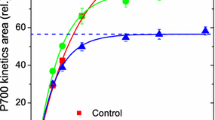
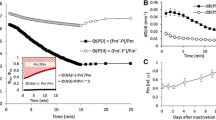
The role of electron transport to O2 in mitigating against photoinactivation of Photosystem (PS) II was investigated in leaves of pea (Pisum sativum L.) grown in moderate light (250 μmol m−2 s−1). During short-term illumination, the electron flux at PS II and non-radiative dissipation of absorbed quanta, calculated from chlorophyll fluorescence quenching, increased with increasing O2 concentration at each light regime tested. The photoinactivation of PS II in pea leaves was monitored by the oxygen yield per repetitive flash as a function of photon exposure (mol photons m−2). The number of functional PS II complexes decreased nonlinearly with increasing photon exposure, with greater photoinactivation of PS II at a lower O2 concentration. The results suggest that electron transport to O2, via the twin processes of oxygenase photorespiration and the Mehler reaction, mitigates against the photoinactivation of PS II in vivo, through both utilization of photons in electron transport and increased nonradiative dissipation of excitation. Photoprotection via electron transport to O2 in vivo is a useful addition to the large extent of photoprotection mediated by carbon-assimilatory electron transport in 1.1% CO2 alone.
Similar content being viewed by others
Abbreviations
- Fm, Fo, Fv-:
-
maximal, initial (corresponding to open PS II traps) and variable chlorophyll fluorescence yield, respectively
- NPQ-:
-
non-photochemical quenching
- PS-:
-
photosystem
- QA-:
-
primary quinone acceptor
- qP-:
-
photochemical quenching coefficient
References
Aro E-M, McCaffery S, and Anderson JM (1993) Photoinhibition and D1 protein degradation in peas acclimated to different growth irradiances. Plant Physiol 103: 835–843
Barber J (1995) Molecular basis of the vulnerability of Photosystem II to damage by light. Aust J Plant Physiol 22: 201–208
Baroli I and Melis A (1996) Photoinhibition and repair in Dunaliella salina acclimated to different growth irradiances. Planta 198: 640–646
Bell CJ and Rose DA (1981) Light measurement and the terminology of flow. Plant Cell Environ 4: 89–96
Björkman O and Demmig B (1987) Photon yield of O2 evolution and chlorophyll fluorescence at 77 K among vascular plants of diverse origins. Planta 170: 489–504
Chow WS (1994) Photoprotection and photoinhibitory damage. Adv Mol Cell Biol 10: 151–196
Chow WS, Hope AB, and Anderson JM (1991) Further studies on quantifying Photosystem II in vivo by flash-induced oxygen yield from leaf discs. Aust J Plant Physiol 18: 397–410
Cornic G and Briantais J-M (1991) Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta 183: 178–184
Demmig-Adams B and AdamsIII WW (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43: 599–626
Foyer CH, Lelandais M and Kunert KJ (1994) Photooxidative stress in plants. Physiol Plant 92: 696–717
Grace SC and Osmond CB (1995) H2O2 dehydrogenase quenching of fluorescence in thylakoids and chloroplasts: the prime role of thylakoid ascorbate peroxidase in electron transport to O2. In: Mathis P (ed) Photosynthesis: From Light to Biosphere, Vol IV, pp 383–388. Kluwer Academic Publishers, Dordrecht
Greer DH, Berry JA, and Björkman O (1986) Photoinhibition of photosynthesis in intact bean leaves: role of light and temperature and requirement for chloroplast-protein synthesis during recovery. Planta 168: 253–260
Havaux M and Davaud A (1994) Photoinhibition of photosynthesis in chilled potato leaves is not correlated with a loss of Photosystem II activity. Photosynth Res 40: 75–92
Horton P, Ruban AV and Walters RG (1996) Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 47: 655–684
Jones LW and Kok B (1966) Photoinhibition of chloroplasts reactions. Kinetics and action spectra. Plant Physiol 41: 1037–1043
Keren N, Gong H and Ohad I (1995) Oscillations of reaction center II-D1 protein degradation in vivo induced by repetitive light flashes. J Biol Chem 270: 806–814
Long SP, Humphries S and Falkowski PG (1994) Photoinhibition of photosynthesis in nature. Annu Rev Plant Physiol Plant Mol Biol 45: 633–662
Miyake C and Asada K (192) Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol 33: 541–553
Miyake C and Asada K (1994) Ferredoxin-dependent photoreduction of the monodehydroascorbate radicals in spinach thylakoids. Plant Cell Physiol 35: 539–549
Nagy L, Balint E, Barber J, Ringler A, Cook KM and Maroti P (1995) Photoinhibition and law of reciprocity in photosynthetic reactions of Synechocystis sp. PCC 6803. J Plant Physiol 145: 410–415
Öquist G, Chow WS and Anderson JM (1992) Photoinhibition of photosynthesis represents a mechanism for the long-term regulation of Photosystem II. Planta 186: 450–460
Öquist G and Huner NPA (1993) Cold-hardening-induced resistance to photoinhibition of photosynthesis in winter rye is dependent upon an increased capacity for photosynthesis. Planta 189: 150–156
Osmond CB (1981) Photorespiration and photoinhibition. Some implications for the energetics of photosynthesis. Biochim Biophys Acta 639: 77–98
Osmond CB (1994) What is photoinhibition? Some insights from comparisons of shade and sun plants. In: Baker NR and Bowyer JR (eds) Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field, pp 1–24. Bios Scientific Pub, Oxford
Osmond CB and Grace SC (1995) Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis? J Exp Bot 46: 1351–1362
Park Y-I, Anderson JM and Chow WS (1996a) Photoinactivation of functional Photosystem II and D1 protein synthesis in vivo are independent of the modulation of the photosynthetic apparatus by growth irradiance. Planta 198: 300–309
Park Y-I, Chow WS and Anderson JM (1995a) Light inactivation of functional Photosystem II in leaves of peas grown in moderate light depends on photon exposure. Planta 196: 401–411
Park Y-I, Chow WS and Anderson JM (1995b) The quantum yield of photoinactivation of Photosystem II in pea leaves is greater at low than high photon exposure. Plant Cell Physiol 36: 1163–1167
Park Y-I, Chow WS and Anderson JM (1995c) Electron transport to oxygen protects Photosystem II against light stress. In: Mathis P (ed) Photosynthesis: From Light to Biosphere, Vol IV, pp 393–396. Kluwer Academic Publishers, Dordrecht
Park Y-I, Chow WS, Anderson JM and Hurry VM (1996b) Differential susceptibility of Photosystem II to light stress in light-acclimated pea leaves depends on the capacity for phototchemical and non-radiative dissipation of light. Plant Sci 115: 137–149
Porra RJ, Thompson WA and Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b with four different solvents: verification of the concentration of chlorophyll by atomic absorption spectroscopy. Biochim Biophys Acta 975: 384–394.
Radmer RJ and Kok B (1976) Photoreduction of O2 primes and replaces CO2 assimilation. Plant Physiol 58: 336–340
Schreiber U and Neubauer C (1990) O2-dependent electron flow, membrane energization and the mechanism of nonphotochemical quenching of chlorophyll fluorescence. Photosynth Res 25: 279–293
Schreiber U, Bilger W and Neubauer C (1994) Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze ED and Caldwell M (eds) Ecophysiology of Photosynthesis, pp 49–70. Springer-Verlag, Berlin
Sonoike K and Terashima I (1994) Mechanism of Photosystem-I photoinhibition in leaves of Cucumis sativus L. Planta 194: 287–293
Tyystjärvi E and Aro E-M (1996) The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc Natl Acad Sci USA 93: 2213–2218
Vavalin DV, Tyystjäärvi E and Aro EM (1995) In search of a reversible stage of photoinhibition in a higher plant: No changes in the amount of functional Photosystem II accompany relaxation of variable fluorescence after exposure of lincomycin-treated Cucurbita pepo leaves to high light. Photosynth Res 45: 239–247
Walker D (1992) Excited leaves. New Phytol 121: 325–345
Wu J, Neimanis S and Heber U (1991) Photorespiration is more effective than the Mehler reaction in protecting photosynthetic apparatus against photoinhibition. Bot Acta 104: 283–291
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Park, YI., Chow, W.S., Osmond, C.B. et al. Electron transport to oxygen mitigates against the photoinactivation of Photosystem II in vivo . Photosynth Res 50, 23–32 (1996). https://doi.org/10.1007/BF00018218
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00018218