Abstract
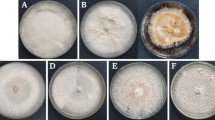
The ability of endophytic bacteria to influence Erwinia carotovora var. atroseptica (Eca) growth and disease development was examined in potatoes. Bacterial populations isolated from within the tubers of five potato (Solanum tuberosum L.) cultivars (Kennebec, Butte, Green Mountain, Russet Burbank and Sebago) showed antibiosis toward Eca in an in vitro assay. Sebago was host to the highest percentage of bacterial isolates inhibiting Eca growth in vitro (49.5%), followed by Green Mountain (33.3%), Kennebec (29.3%), Russet Burbank (12.9%) and Butte (1.8%). Of these, Curtobacterium luteum was the most common species. Few endophytic bacteria from Butte were inhibitory to Erwinia; all were from Pantoea agglomerans. Significantly higher populations of Erwinia-inhibiting bacteria were recovered from Kennebec (1.89 × 106 cfu fresh weight tuber tissue) as compared to the other cultivars; the lowest populations were recovered from Butte (0.01 × 106 cfu per g fresh weight tuber tissue). Published levels of cultivar disease resistance to blackleg did not correspond to actual bacterial soft rot development (induced by Eca) in an in vivo (tuber) assay. However, bacterial soft rot development was negatively correlated with the density of tuber populations of endophytic bacteria found able to inhibit Eca growth in vitro (R=−0.879, p=0.05).
Similar content being viewed by others
References
Anon 1994 Potato crop, variety, weed and pest control recommendations 1994, for the Atlantic Provinces. Prepared by the Advisory Committee on Potatoes Agdex 257, Publication 1300A, ISSN 0836–947X, Canada.
Bergey 1994 Bergey's Manual of Determinative Bacteriology 1994. Eds. J GHolt, N RKreig, P HSneath, J TStal and S TWilliams. 9th edition. Williams and Wilkins, Baltimore, MD, USA.
Chanway C P, Nelson L M and Holl F B 1988a Cultivar-specific growth promotion of spring wheat (Triticum aestivum L.) by coexistant Bacillus species. Can. J. Microbiol. 34, 925–929.
Chanway C P, Holl F B and Turkington R 1988b Genotypic coadaption in plant growth promotion of forage species by Bacillus polymyxa. Plant and Soil 106, 281–284.
Chen C Q, Musson G, Bauske E and Kloepper J W 1993 Potential of endophytic bacteria for biological control of Fusarium Wilt of Cotton. Phytopathology 83, 1404.
Clark F E 1965 Agar-plate method for total microbial count. Chapter 99. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties. Eds. C ABlack, D DEvans, J LWhite, L EEnsminger and F EClark. pp 1460–1466. Am. Soc. Agronomy Inc., Madison, WI, USA.
Colyer P D and Mount M S 1984 Bacterization of potatoes with Pseudomonas putida and its influence on postharvest soft rot diseases. Plant Dis. 68, 703–706.
Genstat 1993 Genstat 5 (Release 3). Genstat 5 Committee of the Statistics Department. Eds. R WPayne, P WLane, P G NDigby, S AHarding, P KLeech, G WMorgan, A DTodd, RThompson, GTunnicliffe Wilson, S JWelham and R PWhite. Rothampstead Experimental Station. Oxford Science Publications, Clarendon Press, Oxford, UK.
Glandorf D C M, Peters L G L, Van derSluis I, Bakker P A H M and Schippers B 1993 Crop specificity of rhizosphere pseudomonads and the involvement of root agglutins. Soil Biol. Biochem. 25, 981–989.
Kloepper J W 1983 Effect of seed piece inoculation with plant-growth promoting rhizobacteria on populations of Erwinia carotovora on potato roots and daughter tubers. Phytopathology 73, 217–219.
Kloepper J W and Schroth M N 1981 Relationship of in vitro antibiosis of plant growth promoting rhizobacteria to plant growth and the displacement of root microflora. Phytopathology 71, 1020–1024.
Kloepper J W, Schroth M N and Miller T D 1980 Effects of rhizosphere colonization by plant growth-promoting rhizobacteria on potato plant development and yield. Phytopathology 70, 1078–1082.
Leyns F, Lambert B, Joos H and Swings J 1990 Antifungal bacteria from different crops. In Biological Control of Soil-borne Plant Pathogens. Ed. DHornby. pp 437–444, CAB International, Wallingford, UK.
Liu L, Kloepper J W and Tuzun S 1995 Induction of systemic resistance in cucumber against Fusarium Wilt by plant growth-promoting rhizobacteria. Phytopathology 85, 695–698.
Loper J E, Haack A and Schroth M N 1984 Population dynamics of soil pseudomonads in the rhizosphere of potato (Solanum tuberosum L.). Appl. Environ. Microbiol. 49, 416–422.
McInroy J A and Kloepper J W 1994 Studies on indigenous endophytic bacteria of sweet corn and cotton. In Molecular Ecology of Rhizosphere Microorganisms. Eds. FO'Gara, D NDowlin and BBoesten. pp 19–28. VCH, Germany.
Nowak J, Asiedu S K, Lazrovits G, Pillay V, Stewart A, Smith C and Liu Z 1995 Enhancement of in vitro growth and transplant stress tolerance of potato and vegetable plantlets co-cultured with a plant growth promoting pseudomonad bacterium. In Ecophysiology and Photosynthetic In Vitro Cultures. Eds. F Carre and P Chagvardieff. pp 173–180. Proceedings of the International Symposium, Aix-en-Provence, France.
Old K M and Nicolson T H 1978 The root cortex as part of the microbial continuum. In Microbial Ecology. Eds. M WLoutit and J A RMiles. pp 291–294. Springer-Verlag, New York, USA.
Pérombelon M C M and Kelman A 1980 Ecology of the Soft Rot Erwinias. Annu. Rev. Phytopathol. 18, 361–387.
Schaad N W 1988 Initial identification of common genera. In A laboratory Guide for the Identification of Plant Pathogenic Bacteria. 2nd edition. Ed. N WSchaad. pp 1–14. APS Press, St. Paul, MN, USA.
Sturz A V 1995 The role of endophytic bacteria during seed piece decay and potato tuberization. Plant and Soil 175, 257–263.
Sturz A V and Christie B R 1995 Endophytic bacterial systems governing red clover growth and development. Ann. Appl. Biol. 126, 285–290.
VanBuren A M, Andre C and Ishimaru C A 1993 Biological control of the bacterial ring rot pathogen by endophytic bacteria isolated from potato. Phytopathology 83, 1406.
VanPeer R, Neiman G J and Schippers B 1991 Induced resistance and phytoalexin accumulation in biological control of fusarium wilt of carnation by Pseudomonas sp. strain WCS417r. Phytopathology 81, 728–734.
VanPeer R, Punte H L M, deWeger L A and Schippers B 1991 Characterization of root surface and endorhizosphere pseudomonads in relation to their colonization of roots. Appl. Environ. Microbiol. 56, 2462–2470.
Xu G W and Gross D C 1986 Selection of fluorescent pseudomonads antagonistic to Erwinia caratovora and suppressive of potato seed piece decay. Phytopathology 76, 412–422.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sturz, A.V., Matheson, B.G. Populations of endophytic bacteria which influence host-resistance to Erwinia-induced bacterial soft rot in potato tubers. Plant Soil 184, 265–271 (1996). https://doi.org/10.1007/BF00010455
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00010455