Abstract
Three field experiments were performed in Lake Lacawac, PA to determine the importance of potentially limiting nutrients relative to other factors (grazing, depth) in structuring shallow water algal periphyton communities. All three experiments measured periphyton growth (as chlorophyll-a, AFDM or biovolumes of the algal taxa) on artificial clay flower pot substrates which released specified nutrients to their outer surfaces.
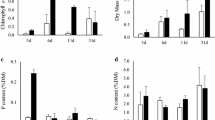
Control of standing crop by nutrient supply rate vs. grazing was examined in Expt. I. Substrates releasing excess N and P, together with one of 4 levels of C (as bicarbonate) were placed either inside or outside exclosures designed to reduce grazer densities. Chlorophyll-a rose from 1.1–25.6 µg.cm−2, and some dominant taxa (e.g., Oedogonium, Nostoc, Anacystis) were replaced by others (e.g., Scenedesmus, Cryptomonas) as bicarbonate supply increased. Reductions in invertebrate density did not significantly affect chlorophyll-a at any of the nutrient levels.
Reasons for the species shift were further evaluated in Expt. II, using a minielectrode to measure the elevation of pH within the periphyton mat through photosynthetic utilization of bicarbonate. The pH adjacent to pots diffusing N, P and large quantities of bicarbonate, and supporting high chlorophyll-a densities of 32 µg cm−2, averaged 10.0 compared to 6.3 in the water column. Pots diffusing only N and P supported 0.7 µg chlorophyll-a cm−2 and elevated pH to 8.2. We suspect that bicarbonate addition favored efficient bicarbonate users (e.g., Scenedesmus), while inhibiting other taxa (e.g., Oedogonium) because of the attendant high pH.
Expt. III was designed to test effects of depth (0.1 m vs. 0.5 m) and N (NH4 + vs. NO3 −) upon the growth response to bicarbonate observed in Expts. I and II. Similar standing crop and species composition were noted on pots at 0.1 m vs. 0.5 m. Enrichment with NH4 + vs. NO3 − also appeared to have little effect upon the periphyton community.
Shallow water periphyton communities in Lake Lacawac, when supplied with sufficient N and P, appear to show a distinctive response to increasing bicarbonate concentration and pH which is robust to moderate variation in grazer densities, distance from the water surface, and the form of N enrichment.
Similar content being viewed by others
References
APHA, 1980. Standard Methods for the examination of water and wastewater. 15th ed. American Public Health Association, Washington, DC. 1134 pp.
Allen, E. D. & D. H. N. Spence, 1981. The differential ability of aquatic plants to utilize the inorganic carbon supply in fresh waters. New Phytol. 87: 269–283.
Cattaneo, A., 1983. Grazing on epiphytes. Limnol. Oceanogr. 28: 24–132.
Cattaneo, A. & J. Kalff, 1986. The effect of grazer size manipulation on periphyton communities. Oecologia 69: 612–517.
Cuker, B. E., 1983. Grazing and nutrient interactions in controlling the activity and composition of the epilithic algal community of an arctic lake. Limnol. Oceanogr 25: 133–141.
DeNoyelles, F., Jr. & W. J. O'Brien, 1978. Phytoplankton succession in nutrient enriched experimental ponds as related to changing carbon, nitrogen and phosphorus conditions. Arch. Hydrobiol. 84: 137–165.
Evans, D. & J. G. Stockner, 1972. Attached algae on artificial and natural substrates in Lake Winnipeg, Manitoba. J. Fish. Res. Bd. Can. 29: 31–44.
Fairchild, G. W., 1981. Movement and microdistribution of Sida crystallina and other littoral microcrustacea. Ecology 62: 1341–1352.
Fairchild, G. W. & A. E. Everett, 1988. Nutrient (N, P, C) limitation of algal periphyton standing crop, species composition and primary production in an oligotrophic softwater lake. Freshw. Biol. 19: 57–70.
Fairchild, G. W., R. L. Lowe & W. B. Richardson, 1985. Algal periphyton growth on nutrient-diffusing substrates: An in situ bioassay. Ecology 66: 465–472.
Gannon, J. E., 1971. Two counting cells for the enumeration of zooplankton microcrustacea. Trans. Am. Micros. Soc. 90: 486–490.
Goulden, C. E., 1971. Environmental control of the abundance and distribution of the chydorid Cladocera. Limnol. Oceanogr. 16: 320–331.
Grahn, O., H. Hultberg, & L. Lander, 1974. Oligotrophication — a self-accelerating process in lakes subjected to excessive supply of acid substances. Ambio 3: 93–94.
Hendrey, G. R., K. Baalsrud, T. S. Traaen, M. Laake & G. Raddum, 1976. Acid Precipitation: Some hydrobiological changes. Ambio 5: 224–227.
Jorgensen, B. B., N. P. Revsbech, & Y. Cohen, 1983. Photosynthesis and structure of benthic microbial mats: microelectrode and SEM studies of four cyanobacterial communities. Limnol. Oceanogr. 28: 1075–1093.
Lazarek, S., 1985. Epiphytic algal production in the acidified Lake Gardsjon, SW Sweden. Ecol. Bull. 37: 213–218.
Lazarek, S., 1986. Responses of the Lobelia-epiphyte complex to liming of an acidified lake. Aquat. Bot. 25: 73–82.
Maberly, S. C., 1983. The interdependence of photon irradiance and free carbon dioxide or bicarbonate concentration on the photosynthetic compensation points of freshwater plants. New Phytol. 93: 1–12.
Mason, C. F. and R. J. Bryant, 1975. Periphyton production and grazing by chironomids in Alderfen Broad, Norfolk. Freshwat. Biol. 5: 271–277.
Müller, P., 1980. Effects of artificial acidification on the growth of periphyton. Can. J. Fish. Aquat. Sci. 37: 355–63.
Nerozzi, A. & P. Siver, 1984. Periphytic community analysis in an oligotrophic lake. Proc. Penna. Acad. Sci. 57: 138–142.
Nusch, E. A., 1980. Comparison of different methods for chlorophyll and phaeopigment determination. Arch. Hydrobiol. 14: 14–36.
Ohle, W., 1981. Photosynthesis and chemistry of an extremely acidic bathing pond in Germany. Verh. int. Ver. Limnol. 21: 1172–1177.
Økland, J. & K. A. Økland, 1986. The effects of acid deposition on benthic animals in lakes and streams. Experientia 42: 471–186.
Palmer, C. M. & P. C. Maloney, 1954. A new counting slide for counting nannoplankton. Spec. Publ. No. 21, Am. Soc. Limnol. Oceanogr., 7 pp.
Phoenix, D. R., 1976. Temporal dynamics of a natural multipredator-multiprey system. PhD Thesis, University of Pennsylvania.
Redfield, A. C., 1958. The biological control of chemical factors in the environment. Am. Scientist 46: 205–221.
Rigano, V. D. M., V. Vona, C. D. Martino & C. Rigano, 1986. Effect of darkness and CO2 starvation on NH4 + and NO3 − assimilation in the unicellular alga Cyanidium caldarium. Physiol. Plant. 68: 34–38.
SAS Institute, 1982. SAS User's Guide: Statistics. SAS Institute, Inc., Cary, NC.
Schindler, D. W., 1980. Experimental acidification of a whole lake: a test of the oligotrophication hypothesis. In D. Drablos & A. Tollan (eds) Ecological Impact of Acid Precipitation. OSLO SNSF Project: 370–374.
Schindler, D. W., 1986. The significance of in-lake production of alkalinity. Wat. Air. Soil Pollut. 30: 931–944.
Schindler, D. W., D. R. S. Lean & E. J. Fee, 1973. Nutrient cycling in freshwater ecosystems. In D. E. Reichle, J. F. Franklin & D. W. Goodall (eds) Productivity of World Ecosystems. National Academy of Sciences: 96–105.
Siver, P. A. & J. S. Chock, 1986. Phytoplankton dynamics in a chrysophycean lake. In J. Kristiansen & R. A. Andersen (eds) Chrysophytes: Aspects and Problems. Cambridge University Press: 165–183.
Stevenson, R. J., R. Singer, D. A. Roberts & C. W. Boylen, 1985. Patterns of epipelic algal abundance with depth, trophic status and acidity in poorly buffered New Hampshire lakes. Can. J. Fish. Aquat. Sci. 42: 1501–1512.
Stokes, P. M., 1984. pH-related changes in attached algal communities of softwater lakes. In G. R. Hendrey (ed.) Early Biotic Responses to Advancing Lake Acidification. Butterworth, Stoneham, MA: 43–61.
Stumm, W. & S. J. J. Morgan, 1970. Aquatic Chemistry. Wiley Interscience, New York, NY. 583 pp.
Tessier, A. J., 1981. The comparative population regulation of two planktonic Cladocera: Holopedium giberrum and Daphnia catawba. PhD Thesis. University of Pennsylvania.
Toetz, D., 1971. Diurnal uptake of NO3 − and NH4 + by a Ceratophyllum-periphyton community. Limnol. Oceanogr. 16: 819–822.
Turner, M. A., D. W. Schindler & R. W. Graham, 1983. Photosynthesis-irradiance relationships of epilithic algae measured in the laboratory and in situ. In R. G. Wetzel (ed.) Periphyton of Freshwater Ecosystems. Dr. W. Junk Publishers, The Hague, Netherlands: 73–87.
Wilcox, G. R. & J. DeCosta, 1984. Bag experiments on the effects of phosphorus and base additions on the algal biomass and species composition of an acid lake. Int. Rev. ges. Hydrobiol. 69: 173–199.
Young, O. W., 1945. A limnological investigation of periphyton in Douglas Lake, MI. Trans. Am. Micros. Soc. 64: 1–20.
Zar, J. H., 1984. Biostatistical Analysis. 2nd Ed. Prentice-Hall, Inc., Englewood Cliffs, NJ. 718 pp.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fairchild, G.W., Sherman, J.W. & Acker, F.W. Effects of nutrient (N, P, C) enrichment, grazing and depth upon littoral periphyton of a softwater lake. Hydrobiologia 173, 69–83 (1989). https://doi.org/10.1007/BF00008600
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00008600