Abstract
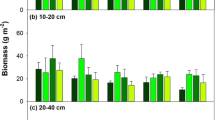
Root production and turnover were studied using sequential core sampling and observations in permanent minirhizotrons in the field in three dry heathland stands dominated by the evergreen dwarfshrub Calluna vulgaris and the grasses Deschampsia flexuosa and Molinia caerulea, respectively. Root biomass production, estimated by core sampling, amounted to 160 (Calluna), 180 (Deschampsia) and 1380 (Molinia) g m-2 yr-1, respectively. Root biomass turnover rate in Calluna (0.64 yr-1) was lower compared with the grasses (Deschampsia: 0.96 yr-1; Molinia 1.68yr-1)). Root length turnover rate was 0.75–0.77 yr-1 (Deschampsia) and 1.17–1.49 yr-1 (Molinia), respectively. No resorption of N and P from senescing roots was observed in either species. Input of organic N into the soil due to root turnover, estimated using the core sampling data, amounted to 1.8 g N m-2 yr-1(Calluna), 1.7 g N m-2 yr-1 (Deschampsia) and 19.7 g N m-2 yr-1 (Molinia), respectively. The organic P input was 0.05, 0.07 and 0.55 g P M-2 yr-1, respectively. Using the minirhizotron turnover estimates these values were20–22% (Deschampsia) and 11–30% (Molinia) lower.
When the biomass turnover data were used, it appeared that in the Molinia stand root turnover contributed 67% to total litter production, 87% to total litter nitrogen loss and 84% to total litter phosphorus loss. For Calluna and Deschampsia these percentages were about three and two times lower, respectively.
This study shows that (1) Root turnover is a key factor in ecosystem C, N, and P cycling; and that (2) The relative importance of root turnover differs between species.
Similar content being viewed by others
References
Aber JD, Melillo JM, Nadelhoffer KJ, McClaugherty CA & Pastor J (1985) Fine root turnover in forest ecosystems in relation to quantity and form of nitrogen availability: a comparison of two methods. Oecologia 66: 317–321
Aerts R (1989) Aboveground biomass and nutrient dynamics of Calluna vulgaris and Molinia caerulea in a dry heathland. Oikos 56: 31–38
Aerts R (1990) Nutrient use efficiency in evergreen and deciduous species from heathlands. Oecologia 84: 391–397
Aerts R, Berendse F, Klerk NM & Bakker C (1989) Root production and root turnover in two dominant species of wet heathlands. Oecologia 81: 374–378
Aerts R, Berendse F, De Caluwe H & Schmitz M (1990) Competition in heathland along an experimental gradient of nutrient availability. Oikos 57: 310–318
Berendse F, Beltman B, Bobbink R, Kwant R & Schmitz M (1987) Primary production and nutrient availability in wet heathland ecosystems. Acta Oecol. /Oecol. Plant. 8 (22): 265–279
Berendse F, Bobbink R & Rouwenhorst G (1989) A comparative study on nutrient cycling in wet heathland ecosystems II. Litter decomposition and nutrient mineralization. Oecologia 78: 338–348
Böhm W (1979) Methods of studying root systems. Springer-Verlag, Berlin
Caldwell MM (1979) Root structure: the considerable cost of belowground function. In: Solbrig OT, Jain S, Johnson GB & Raven PH (Eds) Topics in plant population biology (pp 408–427). Columbia University Press, New York
Chapman SB (1979) Some interrelationships between soil and root respiration in lowland Calluna heathland in southern England. J. Ecol. 67: 1–20
Cheng W, Coleman DC & Box JE Jr. (1990) Root dynamics, production and distribution in agroecosystems on the Georgia Piedmont using minirhizotrons. J. Appl. Ecol. 27: 592–604
Chapin FS (1980) The mineral nutrition of wild plants. Ann. Rev. Ecol. Syst. 11: 233–260
De Smidt JT (1977) Heathland vegetation in the Netherlands. Phytocoenologia 4: 258–316
Forrest GI (1971) Structure and production of North Pennine blanket bog vegetation. J. Ecol. 59: 453–479
French DD (1988) Some effects of changing soil chemistry on decomposition of plant litters and cellulose on a Scottish moor. Oecologia 75: 608–618
Frissel MJ (1981) The definition of residence time in ecological models. In: Clark FE & Rosswall T (Eds) Terrestrial Nitrogen Cycles (pp 117–122). Ecol. Bull. (Stockholm) 33
Grime JP (1979) Plant strategies and vegetation processes. John Wiley and Sons, New York
Kubiena WL (1953). The soils of Europe. Murby, London
McClaugherty CA, Aber JD & Melillo JM (1982) The role of fine roots in the organic matter and nitrogen budgets of two forested ecosystems. Ecology 63: 1481–1490
Persson H (1978) Root dynamics in a young Scots pine stand in Central Sweden. Oikos 30: 508–519
Persson H (1979) Fine-root production, mortality and decomposition in forest ecosystems. Vegetatio 41: 101–109
Raich JW & Nadelholffer KJ (1989) Belowground carbon allocation in forest ecosystems: global trends. Ecology 70: 1346–1354
Robinson D & Rorison IH (1983) A comparison of the responses of Lolium perenne L., Holcus lanatus L. and Deschampsia flexuosa (L.) Trin. to a localized supply of nitrogen. New Phytol. 94: 263–273
Robinson D & Rorison IH (1988) Plasticity in grass species in relation to nitrogen supply. Funct. Ecol. 2: 249–257
SAS Institut Inc. (1985) SAS/STAT Guide for personal computers, Version 6 edition. Cary, N.C.
Singh JS, Lauenroth WK, Hunt HW & Swift DM (1984) Bias and random errors in estimates of net root production: a simulation approach. Ecology 65: 1760–1764
Taylor HM (1987) Minirhizotron observation tubes: Methods and applications for measuring rhizosphere dynamics. ASA Special Publication 50. ASA/CSSA/SSSA, Madison, 143 pp
Tennant D (1975) A test of a modified line intersect method of estimating root length. J. Ecol. 63: 995–1001
Tinhout A & Werger MJA (1988) Fine roots in a dry Calluna heathland. Acta Bot. Neerl. 37: 225–230
Vogt KA & Bloomfield J (1991) Tree root turnover and senescence. In: Waisel Y, Eschel A & Kafkafi U (Eds) Plant roots, the hidden half (pp 287–306). Marcel Dekker Inc, New York
Vogt KA, Grier CC, Vogt DJ (1986) Production, turnover, and nutrient dynamics of above-and belowground detritus of world forests. Adv. Ecol. Res. 15: 303–377
Vogt KA, Grier CC, Meier CE & Keyes MR (1983) Organic matter and nutrient dynamics in forest floors of young and mature Abies amabilis stands in western Washington, as affected by fine root input. Ecological Monographs 53: 139–157
Vos J & Groenwold J (1983) Estimation of root densities by observation tubes and endoscope. Plant Soil 74: 295–300
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aerts, R., Bakker, C. & De Caluwe, H. Root turnover as determinant of the cycling of C, N, and P in a dry heathland ecosystem. Biogeochemistry 15, 175–190 (1992). https://doi.org/10.1007/BF00002935
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00002935