Abstract
Reactive oxygen species (ROS) are an intricate part of normal cellular physiology. In excess, however, ROS can damage all three major classes of macromolecules and compromise cell viability. We briefly discuss the physiology of ROS but focus on the mechanisms cells use to preserve redox homeostasis upon oxidative stress, with particular emphasis on glycolysis. ROS inhibits multiple glycolytic enzymes, including glyceraldehyde 3-phosphate dehydrogenase, pyruvate kinase M2, and phosphofructokinase-1. Consistently, glycolytic inhibition promotes flux into the oxidative arm of the pentose phosphate pathway to generate NADPH. NADPH is critically important, as it provides the reducing power that fuels the protein-based antioxidant systems and recycles oxidized glutathione. The unique ability of pyruvate kinase M2 inhibition to promote serine synthesis in the context of oxidative stress is also discussed.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
Chemical Definition and Sources of ROS
Reactive oxygen species (ROS) is a vague moniker used to describe a variety of oxygen-containing, chemically reactive small molecules, such as superoxide (•O2 −), the hydroxyl radical (HO•), and hydrogen peroxide (H2O2), that cause oxidative stress. ROS can be generated from exogenous sources like ionizing radiation or redox-cycling xenobiotics [1, 2]. Endogenously, ROS are an obligate by-product of aerobic metabolism. Typically, molecular oxygen is reduced by single- or two-electron mechanisms, yielding superoxide or hydrogen peroxide, respectively. Mitochondria are the predominant source of ROS owing to the electron transport chain (ETC), but peroxisomes and the endoplasmic reticulum contribute. During normal respiration, 1–2 % of molecular oxygen is converted to superoxide owing to electron leak at Complexes I and III [1, 3, 4]. Perturbations in mitochondrial metabolism such as changes in oxygen tension and the actions of mitochondrial uncoupling proteins can modulate superoxide production [5, 6]. In addition, enzymes including the NADPH oxidases, which are particularly important in phagocytic cells, xanthine oxidases, uncoupled nitric oxide synthases, and cytochrome P-450s actively produce ROS [7]. Redox-active metal ions, such as iron, can generate the highly reactive hydroxyl radical from hydrogen peroxide via the Fenton reaction [8]. While diverse reactive oxygen species are commonly grouped together under the term ROS, it is important to remember that their chemistry, and hence biology, differ substantially. For instance, hydroxyl radicals react with near diffusion-limited rate constants with almost any organic molecule. The more limited reactivity of hydrogen peroxide enables it to diffuse across membranes and oxidize thiols specifically, thus making it a more suitable ROS second messenger [9, 10]. In general, reactivity comes at the expense of specificity.
Physiology of ROS
In excess, ROS can lead to widespread oxidative damage of all three macromolecular classes—lipids, protein, nucleic acids—and ultimately to cell death via apoptotic or necrotic pathways [11]. For instance, the hydroxyl radical and a protonated form of superoxide can initiate dangerous autocatalytic lipid peroxidation [11–13]. ROS are mutagenic and may therefore promote tumorigenesis [8]. Hydroxyl radical–induced 8-oxoguanine lesions promote genomic G-to-T and C-to-A substitutions due to mismatched base pairing [14]. The hydroxyl radicals produced via ionizing radiation or Fenton reactions are such strong oxidants that they can abstract hydrogen atoms from a polypeptide backbone to generate a carbon radical [8, 15]. In addition, ROS-mediated proline oxidation can result in the cleavage of a protein peptide backbone. Amino acid side chains, such as those of methionine and cysteine and the aromatic groups of phenylalanine, tryptophan, tyrosine, and histidine, are also vulnerable to attack. Protein carbonylation is commonly used as a marker for oxidative stress. Oxidative protein modification can result in protein–protein cross-links. For example, the amino group of a lysine residue can attack a carbonyl of another protein. Importantly, some of the protein oxidative modifications, particularly protein cross-links, are resistant to proteasomal degradation and can inhibit the activity of the proteasome towards other proteins [16].
In moderate amounts, however, ROS are intricately linked with “normal” cellular physiology. In nonphagocytic cells, stimulating tyrosine kinase receptors via epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) induces a transient increase in cellular ROS [8, 17–19]. The signaling can be attenuated by antioxidant treatment. Nature has exploited the redox sensitivity of cysteine thiol groups to develop biochemical switches poised to functionally respond to changes in cellular ROS [20, 21]. Several of these thiol switches respond to growth factor stimulation–induced ROS. Specifically, ROS reversibly inhibits catalytic cysteine residues of the lipid phosphatase PTEN (phosphatase and tensin homolog) by disulfide bond formation and protein tyrosine phosphatases (PTPs) by cyclic sulfonamide formation. Thus, ROS-mediated phosphatase inhibition serves to enhance phosphatidylinositol-3 kinase (PI3K) and tyrosine kinase proliferative and survival signaling [20, 22, 23]. Most cytosolic protein thiol groups have a pKa greater than the physiological pH and are thus protonated and insensitive to the more mild forms of ROS such as hydrogen peroxide. However, the thiol switch local environment significantly reduces the cysteine side chain pKa such that the more nucleophilic thiolate anion predominates [9, 20]. Thus, the thiolate anion is sensitized to changes in cellular ROS and ready to respond. In addition, thiols can react with electrophilic species via a Michael addition mechanism to form a covalent adduct potentially triggering the thiol switch [9].
ROS can both activate and repress transcription factors via thiol switch–based mechanisms. Rather than inhibiting enzymatic activity, as with the phosphatases discussed above, thiol oxidation induces conformational changes to regulate transcription factor subcellular localization. In Saccharomyces cerevisiae, for example, the AP-1-like transcription factor Yap1p responds to oxidative stress via H2O2-induced inter- and intramolecular disulfide exchanges that result in a conformational change in Yap1p. Conformational remodeling masks the nuclear export signal promoting nuclear stabilization and antioxidant gene expression. The Yap1p thiol switch thus permits a yeast cell to regulate an antioxidant gene program that responds to ROS directly [20]. Similarly, mammalian cells utilize a thiol redox switch to induce an antioxidant gene expression program in response to oxidative and xenobiotic stresses. Under “normal” conditions, Keap1 (Kelch-like ECH-associated protein 1) negatively regulates NRF2 (nuclear factor erythroid 2-related factor 2) by acting as an adapter for a CUL3 E3 ligase that targets NRF2 for ubiquitination and proteasomal degradation [24]. Keap1 contains multiple cysteine residues that are targeted by oxidants, including ROS and exogenous or endogenous electrophiles, to disrupt NRF2 repression [25–28]. Thus stabilized, NRF2 can induce expression of approximately 200 genes to promote both antioxidant and xenobiotic responses. Important NRF2 targets include glutathione (GSH) synthesis genes, such as the catalytic (GCLC) and modifier (GCLM) subunits of the rate-limiting step in GSH synthesis, and glutathione reductase (GSR).
Biochemical Mechanisms that Preserve Redox Homeostasis
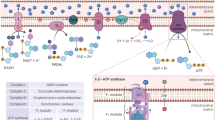
In addition to transcriptional responses like that of NRF2, cells employ a number of strategies to maintain redox homeostasis. The cytosol is maintained at a negative reducing potential of approximately −250 mV using the abundant (1–10 mM) tripeptide glutathione (GSH) and its oxidized form (GSSG) as a redox couple buffer [20]. High-catalytic-activity enzymes rapidly scavenge ROS as they are produced. Cytoplasmic and mitochondrial isoforms of superoxide dismutase (SOD) enhance 10,000-fold the spontaneous dismutation of superoxide to hydrogen peroxide [29]. Peroxisomal catalase (CAT) and glutathione peroxidases (GPx) can further degrade hydrogen peroxide to water and molecular oxygen [10]. Were ROS to evade direct enzymatic scavenging and oxidize protein thiols, the parallel thioredoxin (Trx) and glutaredoxin (Grx) systems reduce the damage. Trx and Grx are small proteins (9–16 kD), which share a dicysteine active site motif (CxxC) in a Trx fold [30]. The Trx mechanism involves a Trx-to-target protein-mixed disulfide that is subsequently nucleophilically attacked, by the remaining active site cysteine, to form an intramolecular Trx disulfide fully reducing the target protein. Grx prefers to attack S-glutathionylated target proteins forming a mixed Grx–glutathione disulfide that is resolved by a second GSH molecule releasing reduced Grx and GSSG. Both systems are ultimately dependent on cellular NADPH-reducing equivalents to regenerate them: Trx reductase (TrxR) and glutathione reductase (GSR) use NADPH to reduce oxidized Trx and GSSG, respectively (Fig. 1) [30]. In addition, glutathione peroxidases such GPx4 use GSH to reduce lipid and cholesterol peroxides [4, 31]. NRF2 activation induces the expression of multiple metabolic enzymes that directly generate NADPH, including glucose-6-phosphate dehydrogenase (G6PD), 6-phosphogluconate dehydrogenase (PGD), isocitrate dehydrogenase (IDH1), and malic enzyme (ME1), while downregulating genes for fatty acid synthesis that consume NADPH [32, 33]. This allows NRF2 to stimulate the production of NADPH, the fundamental source of cellular reducing power. While catalase does not require NADPH for its enzymatic activity, it has an allosteric site for NADPH that maintains catalase in its active conformation [34]. ROS can activate mitogen-activated kinase (MAPK) signaling cascades that respond to cellular stress. Under normal conditions, ASK1 (apoptosis signaling-regulated kinase) is bound to Trx and inhibited. Trx binding requires the Trx dicysteine motif to be reduced. Following oxidation, ASK1 is released and free to oligomerize and autophosphorylate. Thus activated, ASK1 induces MAPK cascades that activate the p38 and JNK stress kinases to promote apoptosis [35]. Interestingly, the α-arrestin family member Trx-interacting protein (TXNIP) seems to integrate glucose availability and ROS. As its name indicates, TXNIP forms intermolecular disulfides with Trx, inhibiting it and promoting oxidative stress [36]. TXNIP furthermore regulates the glucose transporter Glut1 by suppressing Glut1 mRNA and promoting its internalization via clathrin-coated pits. AMP-activated protein kinase (AMPK)—the cellular energy sensor—is activated under low-energy conditions to suppress ATP consumption and increase ATP production. As such, AMPK phosphorylates TXNIP, thereby promoting its degradation via the proteasome to stabilize Glut1 mRNA and maintain Glut1 transporters at the plasma membrane [37].
Antioxidant systems that preserve redox homeostasis. Electron (e−) leak from the electron transport chain (ETC) produces superoxide (•O2 −). Superoxide dismutase (SOD) converts superoxide to hydrogen peroxide. Glutathione peroxidases (GPx) reduce peroxides, such as hydrogen peroxide (H2O2), oxidizing glutathione (GSH) to GSSG. Reactive oxygen species (ROS) can oxidize proteins. The parallel thioredoxin (Trx) and glutaredoxin (Grx) systems can reduce proteins by oxidizing their dicysteine motif or GSH, respectively. Trx reductase (TrxR) and glutathione reductase (GSR) consume NADPH to restore Trx and GSH
Metabolic Adaptations to ROS
Metabolism is profoundly affected by oxidative stress. In excess, oxidation can provoke metabolic failure, compromising cell viability by inactivating enzymes of glycolysis, the Krebs cycle, and the ETC [11, 38]. For example, oxygen-labile iron–sulfur clusters, such as those of aconitase or ETC complexes, are often targeted [4, 39]. However, metabolism has also evolved to respond to such stresses in an adaptive manner. Frequently, the mechanism revolves around thiol-based switches that allow the cell to rewire metabolism in a way that promotes an antioxidant response independent of transcriptional or signaling pathways. As such, metabolism is one of the faster responders; metabolic rewiring is evident within minutes of oxidative stress [40]. We will explore how cells tune glycolytic metabolism to cope with oxidative damage. Much of the antioxidant systems ineluctably rest on the NADPH to NADP+ ratio. Thus, a recurring theme will be how glycolytic flux is diverted into NADPH-generating processes.
The Pentose Phosphate Pathway and NADPH Production
After glucose is imported into the cell via GLUT transporters, it is phosphorylated by hexokinase (HK) at the 6 position to generate glucose-6-phosphate (G6P). Glucose phosphorylation has the dual benefits of trapping glucose within the cell and providing a trans-membrane concentration gradient to draw more glucose in. G6P lies at the nexus of glycolysis, glycogen synthesis—via conversion to glucose-1-phosphate—and the oxidative arm of the pentose phosphate pathway (ox-PPP). The predominant fate of G6P is a function of cell type and metabolic demand. The ox-PPP is traditionally considered the predominant producer of cellular NADPH and is thus critical for antioxidant defense [41]. Conceptually, the ox-PPP is distinct from the reversible non-oxidative phase of the PPP, which does not produce NADPH (Fig. 2) [42]. G6PD catalyzes the first committed and rate-limiting step of the ox-PPP, generating one unit of NADPH and 6-phosphoglucolactone [34]. The unstable lactone ring is opened by phosphogluconolactonase to yield 6-phosphogluconate, which is subsequently decarboxylated by PGD to give an additional unit of NADPH and ribulose-5-phosphate [34, 43]. The net yield per unit of G6P is therefore two NADPH and ribulose-5-phosphate. Ribulose-5-phosphate is an immediate precursor for the ribose-5-phosphate used in the synthesis of nucleotide sugar moieties. The G6P carbon may be recycled back into glycolysis as the non-oxidative arm PPP enzyme transketolase produces the glycolytic intermediates glyceraldehyde-3-phosphate (G3P) and fructose-6-phosphate (F6P) (Fig. 2) [34, 43]. Post-translationally, G6PD is regulated by phosphorylation, protein–protein interaction, and translocation to the plasma membrane upon growth factor stimulation [34, 44–47]. Importantly, G6PD is allosterically activated by the NADP+ to NADPH ratio [34, 48, 49]. Thus, as antioxidant enzymes, including those of the Grx and Trx systems, consume NADPH to reduce ROS-induced damage, NADP+ levels increase, stimulating the activity of the ox-PPP to produce more NADPH and maintain cellular reducing power.
Glycolysis and the pentose phosphate pathway (PPP). The PPP is composed of two distinct arms, the oxidative branch (light blue) and the non-oxidative branch (gray). While both arms produce ribose-5-phosphate, a precursor for nucleotide synthesis, only the oxidative branch concomitantly produces NADPH. Glycolytic flux enters the oxidative branch via glucose-6-phosphate dehydrogenase (G6PD). Fructose-2,6-bisphosphate (F-2,6-BP) activates phosphofructokinase-1 (PFK1) to promote glycolysis (light green). In response to reactive oxygen species (ROS) and UV stress, p53 activates TIGAR (TP53-induced glycolysis and apoptosis regulator). TIGAR degrades F-2,6-BP, thereby inhibiting PFK1. This allows glycolytic flux to be diverted into the oxidative arm and enhances NADPH production to fuel the cellular antioxidant systems. Metabolic enzymes are shown in dark blue
The importance of ox-PPP in protecting against oxidant stress is clearly evident from X-linked G6PD deficiency, the most common human enzyme defect in the world. Erythrocytes are sensitive to oxidative stress and are highly dependent on ox-PPP to maintain NADPH and reduced GSH. Thus, one well-documented and potentially lethal clinical manifestation of G6PD deficiency is acute hemolytic anemia following ingestion of oxidative stress–inducing agents. Such agents include the antimalarial primaquine, sulfonamides, and fava beans. Other patients suffer from chronic anemia [50, 51]. In agreement with the human pathology, in vitro experiments in a variety of cell types show that G6PD inhibition or genetic knockout increases sensitivity to oxidizing agents, including exogenous and endogenous H2O2 [52, 53]. G6PD knockout increases the apoptotic response of CHO cells exposed to ionizing radiation consistent with the role of ROS in apoptosis [54]. Conversely, G6PD overexpression increases resistance to exogenous H2O2 [52, 53]. The combination of human and in vitro data argues that the diversion of glycolytic flux into the ox-PPP pathway plays a vital role in antioxidant defense at both a cellular and organismal level.
Different cell types likely rely on different metabolic pathways to generate their basal level of NADPH. Mutant KRas-driven pancreatic ductal adenocarcinoma cells (PDAC) use glutamine-derived malate to generate basal NADPH, via malic enzyme (ME1), and keep ROS in check. In PDAC, G6PD knockdown does not affect NADPH levels, suggesting that it is not necessary for redox balance [56]. PDAC rely on the non-oxidative PPP branch to promote ribose biogenesis for nucleic acid production, hence decoupling it from NADPH synthesis [57]. In contrast, HEK293T cells are not dependent on ME1 but instead use the ox-PPP and folate cycle to generate basal NADPH and maintain reduced GSH pools [58]. Whether ox-PPP activation from a more inhibited state due to high NADPH levels under “normal” cellular conditions is the predominant NADPH stress response pathway, as some have suggested, needs further investigation [52, 54, 59]. For example, HEK293T cells derive a majority of their NADPH from the ox-PPP with the folate cycle producing a substantial amount [58]. Knockdown of the folate cycle enzymes methylenetetrahydrofolate dehydrogenase 1 (MTHFD1) and MTHFD2 sensitizes HEK293T cells to acute hydrogen peroxide and diamide stress, indicating that the folate cycle also plays a role in dealing with oxidative stress presumably through its substantial NADPH contribution. Whether folate cycle NADPH production is directly activated by ROS stress, like the ox-PPP, remains to be determined. The fact that NRF2 has evolved to regulate the expression of the NADPH-generating enzymes IDH1 and ME1, in addition to G6PD and PGD, suggests that it is beneficial to activate NADPH production not only via the induction of the ox-PPP [28]. In the context of ME1 knockdown PDAC cells, why the increases in NADP+ and ROS do not trigger increased ox-PPP pathway flux directly through G6PD or indirectly via NRF2 is not clear and is surprising given that other cell types are known to do so [53].
Phosphofructokinase-1 Inhibition
Once glucose is trapped within the cell as G6P, it undergoes a reversible isomerization reaction to fructose-6-phosphate (F-6-P) catalyzed by phosphoglucose isomerase (PGI). Phosphofructokinase-1 (PFK1) subsequently phosphorylates F-6-P at the 1 position, yielding fructose-1,6-bisphosphate (F-1,6-BP). Importantly, the PFK1 step is both rate limiting and the first committed step of glycolysis; above PFK1, glycolytic intermediates can enter into glycogen synthesis, the ox-PPP, or the hexosamine pathway [60, 61]. PFK1 functions as the gatekeeper of glycolysis and is therefore highly regulated. ATP and citrate are allosteric inhibitors, while AMP and fructose-2,6-bisphosphate (F-2,6-BP) are activators [60, 61]. The exact PFK1 kinetic parameters are determined by the specific subunit composition [62]. Releasing ATP-based PFK1 inhibition is important to stimulate glucose metabolism in proliferating cells [63]. This is in part achieved by F-2,6-BP-induced PFK1 activation. F-2,6-BP is produced by phosphofructokinase-2 (PFK2) phosphorylating F-6-P at the 2 position (Fig. 2). PFK2 is a bifunctional enzyme containing a kinase domain and bisphosphatase (BPase) domain at the N and C-termini, respectively [64, 65]. Thus, the cellular F-2,6-BP concentration depends on the rates of the two opposing activities. The kinase and BPase activities are regulated transcriptionally and post-translationally via, for example, hormonal stimulation [64, 65]. Conceptually, the F-2,6-BP shunt not only provides a PFK1 feed-forward mechanism to accelerate glycolysis when intracellular F-6-P accumulates but also helps decouple glycolytic flux from the cellular ATP charge. Unsurprisingly, PFK1 and PFK2 are deregulated in cancer [64, 66].
TIGAR (TP53-induced glycolysis and apoptosis regulator) was identified as a p53 target gene induced by ionizing radiation [67, 68]. TIGAR has a single BPase activity that degrades F-2,6-BP to F-6-P [64, 65]. By decreasing F-2,6-BP levels, TIGAR inhibits glycolytic flux downstream of PFK1. PFK1 inhibition allows the G6P and F6P pools to accumulate as their consumption is greatly diminished. The increased G6P can flow into the ox-PPP to generate NADPH. Consistent with this, TIGAR knockdown, or inhibition of upstream positive regulators, leads to increased ROS and a decrease in NADPH and reduced GSH [68–71]. The intestinal crypts of TIGAR knockout mice subjected to whole body irradiation are acutely more apoptotic and have a greater difficulty in regenerating themselves compared with those of wild-type animals [72]. The apoptotic response is suggestive of a failure in dealing with ROS; left unchecked, ROS can trigger apoptosis. Use of an in vitro three-dimensional crypt culture model showed that the TIGAR knockout crypts also have a proliferation defect. The defect can be rescued by exogenous antioxidants or nucleosides. Interestingly, nucleoside addition was found to help sustain a favorable GSH to GSSG ratio [72]. Overall, these mechanisms can be understood in that PFK1 inhibition allows for a buildup of G6P that pushes into the ox-PPP in which a rising NADP+ to NADPH ratio is furthermore activating G6PD. The NADPH thus produced provides reducing power to deal with the oxidative stress. The antioxidant effect of TIGAR under hypoxia is partially independent of its BPase activity and instead depends on TIGAR translocating to the mitochondria and associating with mitochondrial hexokinase-2 [73].
Glyceraldehyde 3-Phosphate Dehydrogenase Inhibition
The redirection of glycolytic flux through the ox-PPP to combat oxidative stress is also achieved by targeting glycolytic enzymes downstream of PFK1. Frequently, the process involves ROS directly oxidizing thiol switches within these enzymes. Subsequent to the PFK1 step, aldolase cleaves F-1,6-BP into two three-carbon molecules: dihydroxyacetone phosphate (DHAP) and G3P. G3P is the substrate of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). GAPDH catalyzes the reversible oxidative phosphorylation of G3P to 1,3-bisphosphoglycerate (1,3-BPG) using NAD+ and inorganic phosphate. 1,3-BPG is a strong product inhibitor of GAPDH [74]. Mechanistically, GAPDH employs a conserved active site cysteine (Cys152 in humans) for a nucleophilic attack on the aldehyde moiety of G3P forming a thiohemiacetal that rearranges to an acyl-enzyme intermediate with a hydride transfer to NAD+. The acyl-enzyme intermediate is resolved by an inorganic phosphate attack [74]. The same active site cysteine involved in catalysis functions as a thiol switch, as discussed below. Interestingly, GAPDH has other enzymatic activities including S-nitrolase, ADP-ribosylase, kinase, and peroxidase [74].
The GAPDH reaction is not at equilibrium and is therefore a potential regulatory point of glycolysis [75]. In mammalian cells, GAPDH is inhibited within minutes of exposure to oxidants predominantly via direct enzyme inactivation and loss of the NAD+ cofactor presumably through PARP activation [40, 76]. The GAPDH active site cysteine is highly sensitive to inhibitory oxidative modifications of ROS and reactive nitrogen oxide species (RNS). With H2O2, the modifications include, in order of increasing oxidation, sulfenic, sulfinic, and sulfonic acid. Additionally, the active site cysteine can oxidize by forming an intramolecular disulfide with a proximal cysteine [20, 40, 77–80].
Beyond direct ROS thiol oxidation, GAPDH is rapidly S-thiolated following both endogenous (e.g., monocyte respiratory bursts) and exogenous oxidative stress. S-thiolation is a posttranslational modification in which proteins form mixed disulfides with low molecular weight thiols. In human cells, the majority of adducts are formed using GSH, but free cysteine also contributes. GAPDH S-thiolation is inhibitory. Activity can be restored by dithioerythritol (DTE) treatment or if the oxidative insult or stimulus is removed, indicating that the inhibition is reversible [81, 82]. S. cerevisiae knockout strains defective in GSH biosynthesis cannot recover GAPDH enzymatic activity, suggesting that GSH is necessary to protect against irreversible thiol hyperoxidation [83]. The process seems to be regulated, because S-thiolation is specific to the Tdh3 isoform of GAPDH in S. cerevisiae, but not the Tdh2 isoform, despite high sequence homology (96 % identity). Tdh3 recovers activity within a 2-h period, but not Tdh2. Interestingly, the isozymes are required to deal with different types of exogenous oxidative stress—lethal dose versus a continuous low-level challenge [84]. ATP levels plummet following ROS stress as both mitochondrial and glycolytic ATP synthesis is inhibited [40, 76]. Protecting GAPDH from irreversible oxidation via S-thiolation may allow a cell to quickly resume glycolysis and hence ATP production after the stress wanes. Without a sufficiently rapid recovery of ATP synthesis, cell death may ensue. Oxidative stress can also induce GAPDH aggregation via intermolecular disulfide bonds dependent on the active site cysteine. Such aggregates are found in brain extracts from Alzheimer’s disease (AD) patients and may participate in the proapoptotic functions of GAPDH [20, 85, 86]. Importantly, GAPDH inhibition helps divert glycolytic flux into the ox-PPP pathway by allowing metabolites to accumulate upstream of the point of inhibition consistent with the observed induction of PPP enzymes following H2O2 treatment (Fig. 3) [83, 87]. Triose phosphate isomerase (TPI) immediately precedes GAPDH in glycolysis. Both Caenorhabditis elegans and S. cerevisiae mutants with reduced TPI activity are resistant to oxidative stress. Using a combination of genetic knockouts of PPP enzymes and metabolomic studies, it was shown that low-TPI-activity mutants or ROS inhibition of GAPDH rerouted flux through the PPP [88, 89]. Thus, GAPDH is an important target of ROS that mediates cellular antioxidant response.
Reactive oxygen species (ROS)-mediated inhibition of glycolysis reroutes flux into the oxidative arm of the pentose phosphate pathway. ROS inactivates glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and the pyruvate kinase isoform PKM2 by directly targeting cysteine residues. Alternatively, ROS and UV stress can trigger p53-dependent TIGAR (TP53-induced glycolysis and apoptosis regulator) activation that inhibits phosphofructokinase-1 (PFK1). Glycolytic inhibition promotes flux into the oxidative pentose phosphate pathway to produce NADPH and fuel cellular antioxidant systems (graded green arrow). For example, NADPH is consumed by glutathione reductase (GSR) to recycle oxidized glutathione (GSSG). PKM2 inhibition is unique in that it allows for a diversion of flux into the serine synthesis pathway. Serine not only contributes to the synthesis of macromolecules but is also a precursor for glutathione (GSH). Serine synthesis is activated by a buildup of 2-phosphoglycerate (2PG), which prevents 3-phosphoglycerate (3PG)-induced inhibition of the oxidative pentose phosphate arm. Enzymes are shown in purple. ROS targets are shown in red
Pyruvate Kinase M2 Inhibition
Pyruvate kinase (PK) catalyzes the final reaction of glycolysis transferring the phosphate moiety of phosphoenolpyruvate (PEP) to ADP, thus generating pyruvate and ATP. Mammals have four PK isoforms. The liver (PKL) and erythrocyte (PKR) isoforms are produced from the PKLR gene. The PKM1 and PKM2 isoforms derive from alternate splicing of exons 9 and 10 of the PKM gene, respectively [90–93]. PKM1 is predominantly expressed in adult differentiated tissues with a high ATP demand, such as the brain, heart, and muscle. PKM2 is expressed over the course of development, in cancers, and in tissues such as the spleen and lungs [94, 95]. PKM2 differs from PKM1 in that it has a lower intrinsic enzymatic activity and has unique regulatory properties. PKM2 allosteric activators include AMP, the de novo purine synthesis intermediate SAICAR (succinylaminoimidazolecarboxamide ribose-5-phosphate), the glycolytic intermediate F-1,6-BP, and the amino acid serine [95–98]. Cellular PKM2 is in a dynamic equilibrium between a less active monomeric form and a more active tetrameric form. Mechanistically, F-1,6-BP allosterically activates PKM2 by stabilizing the tetramer. Conversely, as F-1,6-BP levels drop, the monomeric form prevails, inhibiting PKM2 activity. Thus, F-1,6-BP provides a regulatory loop to coordinate PKM2 activity based on the product of the critical PFK1 step and glucose availability [99–101]. Phosphotyrosine protein binding, tyrosine phosphorylation (Y105), and lysine acetylation (K433) prevent F-1,6-BP binding, thereby inhibiting PKM2 activity [102–104]. Surprisingly, multiple non-glycolytic functions unique to PKM2 have been proposed, including protein kinase and transcriptional coactivator activities. The role of PKM2 in cancer is under intensive study, in part because it has been argued that PKM2 is critical for the metabolic rewiring needed to support cancer cell proliferation, and also because of its novel non-glycolytic activities [96, 102, 105–111]. In studying the glycolytic function of PKM2 in cancer cells, it has become clear that PKM2 contains a thiol switch that is targeted by ROS [112].
Across diverse organisms ranging from Escherichia coli to humans, PK activity is inhibited by oxidative stresses [86, 112–114]. One of the earlier observations was that E. coli PK stored cold for prolonged periods of time without a reducing agent lost activity. Activity was unresponsive to the conventional activators AMP and F-1,6-BP, but could be recovered by incubating the inactive species with the reducing agents beta-mercaptoethanol or dithiothreitol (DTT) [113]. Whether the inhibition was an in vitro artifact or physiologically relevant was unclear. Prompted by the link between oxidative stress and Alzheimers disease (AD), proteomic studies to identify oxidatively modified proteins in the hippocampi of patients suffering from mild cognitive impairment, a condition that commonly progresses to AD, revealed that PKM2 was significantly more carboxylated in those patients than in controls [86]. Interestingly, in S. cerevisiae, low PK activity activates respiration. Despite increased oxidative phosphorylation, increased ROS production is suppressed, hinting at some antioxidant function of low PK activity [115]. PEP functions as a competitive inhibitor of human and yeast TPI. Crystallographic studies indicate that the PKM2 substrate PEP binds directly in the TPI catalytic pocket [115, 116]. Thus, low PK activity enables PEP to accumulate, to form a negative feedback loop that reduces GAPDH substrate availability by preventing the interconversion of DHAP and G3P. TPI inhibition redirects flux into the PPP pathway and protects yeast from a variety of oxidative stresses explaining how the increased respiration resulting from low PK activity does not promote ROS [115]. Previous work had shown that TPI loss-of-function mutants in S. cerevisiae and C. elegans are similarly resistant to exogenous oxidative stresses in a manner genetically dependent on PPP enzymes [88, 89].
Studying PKM2 in the context of cancer cell metabolism not only elucidated the mechanism whereby ROS inactivates PKM2, but also identified the functional significance of PMK2 inhibition [112]. In human cancer cells, several types of oxidative stresses, including H2O2, diamide, and hypoxia, inactivate PKM2. DTT restores PKM2 activity to levels commensurate with those of untreated cells. Neither PKM1 nor heteromers of PKM1 and PKM2 are inhibited by oxidation. Oxidation was shown to directly target Cys358 of PMK2 and decrease the levels of the active tetramer thereby explaining the reduced PKM2 activity. Mutating Cys358 to serine abrogates oxidative stress–induced PKM2 dissociation thus preserving the enzymatic activity under stress. Adding small molecule activators that bind to the PKM2 subunit interface and stabilize the tetrameric form similarly prevent ROS-induced dissociation and loss of PKM2 activity [101, 112]. Functionally, PKM2 inhibition allows cells to increase G6P levels and ox-PPP pathway flux to generate more NADPH and hence preserve reduced GSH and prevent intracellular ROS accumulation (Fig. 3). The ROS inducible PKM2 inhibition not only translates into greater survival when cells are exposed to acute oxidative stress, or chronic ROS stress induced by hypoxia, but also increases the tumorigenic potential of cells in xenografts. Both activator-treated and PKM2C358S mutant cells are defective in their antioxidant response indicating how critical tetramer dissociation is to protect against oxidative stress [112]. ROS-mediated PKM2 inhibition also suggests a mechanism whereby PEP levels can accumulate and inhibit TPI, as in the yeast study described above. PEP inhibition of recombinant human TPI in biochemical assays has been demonstrated [115, 116]. Whether TPI inhibition is necessary for the protective effects of PKM2 inhibition in human cells remains unknown. PKM2 has been reported to interact with the HIF1α and HIF2α transcription factors to promote expression of glycolytic genes (e.g., SLC2A1, LDHA, PDK1) and VEGFA. Thus, PKM2 may also promote ROS detoxification by alleviating tumor hypoxia [117, 118].
De Novo Serine Synthesis
While PKM2 inhibition allows cells to fend off ROS by activating the ox-PPP, it may also help cells deal with more chronic oxidative stress by enabling a buildup of the glycolytic intermediate 3-phosphoglycerate (3PG). 3PG can be diverted into the phosphoserine pathway for de novo serine synthesis [119–121]. Alternatively, serine can be imported from the extracellular space by a variety of transporters, including the commonly expressed ASC system (ASCT1 and ASCT2), that mediate the symport of serine, alanine, or cysteine with sodium [122, 123]. Serine plays a vital role in the antioxidant defense system because it is a precursor for the synthesis of GSH (Fig. 3). The phosphoserine synthesis pathway consists of three sequential reactions: first, 3-phosphoglycerate dehydrogenase (PHGDH) oxidizes 3PG using NAD+ to give 3-phosphohydroxypyruvate (3-PHP); second, the PLP-dependent phosphoserine aminotransferase (PSAT1) transaminates 3-phosphohydroxypyruvate to phosphoserine (PSER) utilizing glutamate as the nitrogen donor; finally, phosphoserine phosphatase (PSPH) hydrolyzes the PSER phosphate group to release serine [119–121]. PHGDH, which catalyzes the first committed step of the pathway, was found to be focally amplified in human tumors, particularly those of the breast and melanoma. Cancer cell lines harboring the amplification, and some non-amplified lines overexpressing PHGDH, are uniquely sensitive to knockdown of any enzyme in the pathway [124, 125]. Although some have speculated, the mechanism by which the phosphoserine pathway promotes tumorigenesis and why extracellular serine is unable to compensate remain to be determined [121]. Interestingly, 3PG is a competitive inhibitor of PGD. Thus, an extensive buildup of 3PG can inhibit ox-PPP NADPH production. 3PG levels are kept sufficiently low via a feedback loop that activates 3PG diversion into the phosphoserine pathway. In glycolysis, 3PG is converted to 2-phosphoglycerate (2PG) by phosphoglycerate mutase 1 (PGAM1). 2PG activates PHGDH to deplete excess 3PG, thereby promoting the synthesis of serine and preventing ox-PPP inhibition [126].
Oxidative stress is known to damage all three principal classes of macromolecules -lipids, nucleic acids, and protein [11]. Macromolecules that cannot be repaired by the cellular antioxidant systems can be replaced by newly synthesized molecules. Serine is an important precursor for de novo macromolecule synthesis. Serine is directly incorporated into proteins and the head groups of certain abundant lipids such as sphingosine and phosphatidylserine [127, 128]. Serine hydroxymethyltransferases (SHMTs) convert serine to glycine in a retro-aldol cleavage reaction concomitantly charging the folate pool with a methylene group. In fact, the SHMT reaction is a major source of one-carbon units for the folate cycle. Glycine and the folate cycle donate carbon for the synthesis of purine and pyrimidines [129]. Thus, by contributing to protein, nucleic acid, and lipid synthesis, serine can help cells recover from oxidative damage to macromolecules.
The importance of serine in dealing with oxidative stress is further highlighted by its contribution to GSH synthesis. GSH is an enzymatically synthesized tripeptide composed of glutamate, cysteine and glycine. Cysteine and glycine can both be produced from serine or imported from the extracellular space. Serine combines with homocysteine in the transsulfuration pathway to yield cystathionine, which is subsequently hydrolyzed to cysteine and homoserine [42]. Glycine is formed from serine via SHMTs as described above. Thus, up to two moles of serine can be consumed per mole of GSH produced. In certain cell types, a large fraction of cytosolic NADPH, comparable to that produced via the PPP, is produced from the oxidation of folate cycle one-carbon units derived from serine via the SHMT reaction [58]. Hence, the conversion of serine to glycine may have the twin benefits of fuelling GSH synthesis and providing the NADPH-reducing power to maintain GSH in its reduced form via glutathione reductase. Alternatively, the NADPH could fuel fatty acid synthesis to aid recovery from lipid oxidation damage [130]. There is significant heterogeneity in the propensity of different cell types to synthesize serine de novo suggesting that the anabolic functions of serine following oxidative stress may similarly diverge across cell types [124, 125].
Conclusion
We have seen that ROS can inhibit glycolysis at multiple nodes. A recurring theme is that the inhibition of glycolysis allows cells to divert flux into the ox-PPP pathway to promote NADPH synthesis and protect against oxidative stress. However, there are also differences depending on the exact point of inhibition. Inhibition at the PKM2 step allows cells to promote flux into the serine synthesis pathway, while PFK1 and GAPDH inhibition does not. Furthermore, both GAPDH and PKM2 inhibition can promote dihydroxyacetone phosphate accumulation, which is an important precursor for the glycerol-3-phosphate shuttle and the synthesis of glycerol needed for triglycerides [88, 115]. As of yet, we only have a limited understanding of what determines which glycolytic node is targeted by ROS and what the advantages are for each. For example, both GAPDH and PKM2 are inhibited by hydrogen peroxide, but is the order of inactivation simply determined by the relative order of the redox potentials of their respective cysteines or are other mechanisms involved [81, 112]? Presumably, GAPDH inhibition overrides PKM2 inhibition, as it is upstream of the latter. One could imagine a hierarchical model where PKM2 responds first to oxidative stress, then GAPDH, and finally PFK1. Given the importance of ROS in tumor development and anticancer therapies, a better understanding of how central metabolism and ROS intertwine could uncover interesting biology and suggest mechanisms to enhance current therapies [1].
References
Gorrini C, Harris IS, Mak TW (2013) Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12:931–947. doi:10.1038/nrd4002
Yoshida T, Goto S, Kawakatsu M et al (2012) Mitochondrial dysfunction, a probable cause of persistent oxidative stress after exposure to ionizing radiation. Free Radic Res 46:147–153. doi:10.3109/10715762.2011.645207
Starkov AA (2008) The role of mitochondria in reactive oxygen species metabolism and signaling. Ann N Y Acad Sci 1147:37–52. doi:10.1196/annals.1427.015
Andreyev AY, Kushnareva YE, Starkov AA (2005) Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 70:200–214
Boveris A, Chance B (1973) The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 134:707–716
Nègre-Salvayre A, Hirtz C, Carrera G et al (1997) A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J 11:809–815. doi:10.1096/fj.1530-6860
Handy DE, Loscalzo J (2012) Redox regulation of mitochondrial function. Antioxid Redox Signal 16:1323–1367. doi:10.1089/ars.2011.4123
Valko M, Rhodes CJ, Moncol J et al (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40. doi:10.1016/j.cbi.2005.12.009
Wall SB, Oh J-Y, Diers AR, Landar A (2012) Oxidative modification of proteins: an emerging mechanism of cell signaling. Front Physiol 3:1–9. doi:10.3389/fphys.2012.00369
Forman HJ, Maiorino M, Ursini F (2010) Signaling functions of reactive oxygen species. Biochemistry 49:835–842. doi:10.1021/bi9020378
Avery SV (2011) Molecular targets of oxidative stress. Biochem J 434:201–210. doi:10.1042/BJ20101695
Wang G, Hong Y, Johnson MK, Maier RJ (2006) Lipid peroxidation as a source of oxidative damage in Helicobacter pylori: protective roles of peroxiredoxins. Biochim Biophys Acta 1760:1596–1603. doi:10.1016/j.bbagen.2006.05.005
Bansal AK, Bilaspuri GS (2009) Antioxidant effect of vitamin E on motility, viability and lipid peroxidation of cattle spermatozoa under oxidative stress. Anim Sci Paper Rep 27:5–14
Jang S, Imlay JA (2007) Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J Biol Chem 282:929–937. doi:10.1074/jbc.M607646200
Stadtman ER (1992) Protein oxidation and aging. Science 257:1220–1224. doi:10.1126/science.1355616
Stadtman ER, Levine RL (2003) Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25:207–218. doi:10.1007/s00726-003-0011-2
Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z (1999) Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 13:9–22. doi:10.1096/fj.1530-6860
Bae YS, Kang SW, Seo MS et al (1997) Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem 272:217–221. doi:10.1074/jbc.272.1.217
Catarzi S, Degl’Innocenti D, Iantomasi T et al (2002) The role of H2O2 in the platelet-derived growth factor-induced transcription of the gamma-glutamylcysteine synthetase heavy subunit. Cell Mol Life Sci 59:1388–1394
Brandes N, Schmitt S, Jakob U (2009) Thiol-based redox switches in eukaryotic proteins. Antioxid Redox Signal 11:997–1014. doi:10.1089/ARS.2008.2285
Groitl B, Jakob U (2014) Thiol-based redox switches. Biochim Biophys Acta 1844:1335–1343. doi:10.1016/j.bbapap.2014.03.007
Xu D, Rovira II, Finkel T (2002) Oxidants painting the cysteine chapel: redox regulation of PTPs. Dev Cell 2:251–252
Leslie NR, Bennett D, Lindsay YE et al (2003) Redox regulation of PI 3‐kinase signalling via inactivation of PTEN. EMBO J 22:5501–5510. doi:10.1093/emboj/cdg513
Kobayashi A, Kang M-I, Okawa H et al (2004) Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 24:7130–7139. doi:10.1128/MCB.24.16.7130-7139.2004
Dinkova-Kostova AT, Holtzclaw WD, Cole RN et al (2002) Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci 99:11908–11913. doi:10.1073/pnas.172398899
Zhang DD, Hannink M (2003) Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol 23:8137–8151. doi:10.1128/MCB.23.22.8137-8151.2003
Adam J, Hatipoglu E, O’Flaherty L et al (2011) Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell 20:524–537. doi:10.1016/j.ccr.2011.09.006
Hayes JD, Dinkova-Kostova AT (2014) The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. TIBS 39:199–218. doi:10.1016/j.tibs.2014.02.002
Forman HJ, Fridovich I (1973) Superoxide dismutase: a comparison of rate constants. Arch Biochem Biophys 158:396–400
Holmgren A, Johansson C, Berndt C et al (2005) Thiol redox control via thioredoxin and glutaredoxin systems. Biochem Soc Trans 33:1375–1377. doi:10.1042/BST20051375
Thomas JP, Maiorino M, Ursini F, Girotti AW (1990) Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation. In situ reduction of phospholipid and cholesterol hydroperoxides. J Biol Chem 265:454–461
Wu KC, Cui JY, Klaassen CD (2011) Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol Sci 123:590–600. doi:10.1093/toxsci/kfr183
Mitsuishi Y, Taguchi K, Kawatani Y et al (2012) Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 22:66–79. doi:10.1016/j.ccr.2012.05.016
Stanton RC (2012) Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life 64:362–369. doi:10.1002/iub.1017
McCubrey JA, Lahair MM, Franklin RA (2006) Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal 8:1775–1789. doi:10.1089/ars.2006.8.1775
Yoshihara E, Masaki S, Matsuo Y et al (2014) Thioredoxin/Txnip: redoxisome, as a redox switch for the pathogenesis of diseases. Front Immunol 4:514. doi:10.3389/fimmu.2013.00514
Wu N, Zheng B, Shaywitz A et al (2013) AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol Cell 49:1167–1175. doi:10.1016/j.molcel.2013.01.035
Cecarini V, Gee J, Fioretti E et al (2007) Protein oxidation and cellular homeostasis: emphasis on metabolism. Biochim Biophys Acta 1773:93–104. doi:10.1016/j.bbamcr.2006.08.039
Gardner PR (2002) Aconitase: sensitive target and measure of superoxide. Meth Enzymol 349:9–23
Cochrane CG (1991) Cellular injury by oxidants. Am J Med 91:23S–30S
Voet D, Voet JG, Pratt CW (2008) Fundamentals of biochemistry. Hoboken, New Jersey, USA
Salway JG (2004) Metabolism at a glance. Blackwell, Malden
Riganti C, Gazzano E, Polimeni M et al (2012) The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med 53:421–436. doi:10.1016/j.freeradbiomed.2012.05.006
Cosentino C, Grieco D, Costanzo V (2011) ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J 30:546–555. doi:10.1038/emboj.2010.330
Jiang P, Du W, Wang X et al (2011) p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol 13:310–316. doi:10.1038/ncb2172
Tian WN, Pignatare JN, Stanton RC (1994) Signal transduction proteins that associate with the platelet-derived growth factor (PDGF) receptor mediate the PDGF-induced release of glucose-6-phosphate dehydrogenase from permeabilized cells. J Biol Chem 269:14798–14805
Pan S, World CJ, Kovacs CJ, Berk BC (2009) Glucose 6-phosphate dehydrogenase is regulated through c-Src-mediated tyrosine phosphorylation in endothelial cells. Arterioscler Thromb Vasc Biol 29:895–901. doi:10.1161/ATVBAHA.109.184812
Holten D, Procsal D, Chang HL (1976) Regulation of pentose phosphate pathway dehydrogenases by NADP+/NADPH ratios. Biochem Biophys Res Commun 68:436–441
Kotaka M, Gover S, Vandeputte-Rutten L et al (2005) Structural studies of glucose-6-phosphate and NADP+ binding to human glucose-6-phosphate dehydrogenase. Acta Crystallogr D Biol Crystallogr 61:495–504. doi:10.1107/S0907444905002350
Cappellini MD, Fiorelli G (2008) Glucose-6-phosphate dehydrogenase deficiency. Lancet 371:64–74. doi:10.1016/S0140-6736(08)60073-2
Luzzatto L, Seneca E (2014) G6PD deficiency: a classic example of pharmacogenetics with on-going clinical implications. Br J Haematol 164:469–480. doi:10.1111/bjh.12665
Pandolfi PP, Sonati F, Rivi R et al (1995) Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J 14:5209–5215
Tian WN, Braunstein LD, Apse K et al (1999) Importance of glucose-6-phosphate dehydrogenase activity in cell death. Am J Physiol 276:C1121–C1131
Tuttle S, Stamato T, Perez ML, Biaglow J (2000) Glucose-6-phosphate dehydrogenase and the oxidative pentose phosphate cycle protect cells against apoptosis induced by low doses of ionizing radiation. Radiat Res 153:781–787. doi:10.1667/0033-7587(2000)153%5B0781:GPDATO%5D2.0.CO;2
Tian WN, Braunstein LD, Pang J et al (1998) Importance of glucose-6-phosphate dehydrogenase activity for cell growth. J Biol Chem 273:10609–10617. doi:10.1074/jbc.273.17.10609
Son J, Lyssiotis CA, Ying H et al (2014) Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496:101–105. doi:10.1038/nature12040
Ying H, Kimmelman AC, Lyssiotis CA et al (2012) Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149:656–670. doi:10.1016/j.cell.2012.01.058
Fan J, Ye J, Kamphorst JJ et al (2014) Quantitative flux analysis reveals folate-dependent NADPH production. Nature 510:298–302. doi:10.1038/nature13236
Tuttle SW, Varnes ME, Mitchell JB, Biaglow JE (1992) Sensitivity to chemical oxidants and radiation in CHO cell lines deficient in oxidative pentose cycle activity. Int J Radiat Oncol Biol Phys 22:671–675
Yalcin A, Telang S, Clem B, Chesney J (2009) Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol 86:174–179. doi:10.1016/j.yexmp.2009.01.003
Jenkins CM, Yang J, Sims HF, Gross RW (2011) Reversible high affinity inhibition of phosphofructokinase-1 by acyl-CoA: a mechanism integrating glycolytic flux with lipid metabolism. J Biol Chem 286:11937–11950. doi:10.1074/jbc.M110.203661
Dunaway GA, Kasten TP, Sebo T, Trapp R (1988) Analysis of the phosphofructokinase subunits and isoenzymes in human tissues. Biochem J 251:677–683
Lunt SY, Vander Heiden MG (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 27:441–464. doi:10.1146/annurev-cellbio-092910-154237
Mor I, Cheung EC, Vousden KH (2011) Control of glycolysis through regulation of PFK1: old friends and recent additions. Cold Spring Harb Symp Quant Biol 76:211–216. doi:10.1101/sqb.2011.76.010868
Lee P, Vousden KH, Cheung EC (2014) TIGAR, TIGAR, burning bright. Cancer Metab 2:1. doi:10.1186/2049-3002-2-1
Yi W, Clark PM, Mason DE et al (2012) Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science 337:975–980. doi:10.1126/science.1222278
Jen K-Y, Cheung VG (2005) Identification of novel p53 target genes in ionizing radiation response. Cancer Res 65:7666–7673. doi:10.1158/0008-5472.CAN-05-1039
Bensaad K, Tsuruta A, Selak MA et al (2006) TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126:107–120. doi:10.1016/j.cell.2006.05.036
Peña-Rico MA, Calvo-Vidal MN, Villalonga-Planells R et al (2011) TP53 induced glycolysis and apoptosis regulator (TIGAR) knockdown results in radiosensitization of glioma cells. Radiother Oncol 101:132–139. doi:10.1016/j.radonc.2011.07.002
Lui VWY, Wong EYL, Ho K et al (2011) Inhibition of c-Met downregulates TIGAR expression and reduces NADPH production leading to cell death. Oncogene 30:1127–1134. doi:10.1038/onc.2010.490
Wanka C, Steinbach JP, Rieger J (2012) Tp53-induced glycolysis and apoptosis regulator (TIGAR) protects glioma cells from starvation-induced cell death by up-regulating respiration and improving cellular redox homeostasis. J Biol Chem 287:33436–33446. doi:10.1074/jbc.M112.384578
Cheung EC, Athineos D, Lee P et al (2013) TIGAR is required for efficient intestinal regeneration and tumorigenesis. Dev Cell 25:463–477. doi:10.1016/j.devcel.2013.05.001
Cheung EC, Ludwig RL, Vousden KH (2012) Mitochondrial localization of TIGAR under hypoxia stimulates HK2 and lowers ROS and cell death. Proc Natl Acad Sci U S A 109:20491–20496. doi:10.1073/pnas.1206530109
Seidler NW (2012) GAPDH: biological properties and diversity. Adv Exp Med Biol 985:21–29
Williamson JR (1965) Glycolytic control mechanisms. I. Inhibition of glycolysis by acetate and pyruvate in the isolated, perfused rat heart. J Biol Chem 240:2308–2321
Hyslop PA, Hinshaw DB, Halsey WA et al (1988) Mechanisms of oxidant-mediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J Biol Chem 263:1665–1675
Leichert LI, Gehrke F, Gudiseva HV et al (2008) Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc Natl Acad Sci U S A 105:8197–8202. doi:10.1073/pnas.0707723105
Maller C, Schröder E, Eaton P (2011) Glyceraldehyde 3-phosphate dehydrogenase is unlikely to mediate hydrogen peroxide signaling: studies with a novel anti-dimedone sulfenic acid antibody. Antioxid Redox Signal 14:49–60. doi:10.1089/ars.2010.3149
Jeong J, Jung Y, Na S et al (2011) Novel oxidative modifications in redox-active cysteine residues. Mol Cell Proteomics 10(3):M110.000513. doi:10.1074/mcp.M110.000513
Schmalhausen EV, Pleten AP, Muronetz VI (2003) Ascorbate-induced oxidation of glyceraldehyde-3-phosphate dehydrogenase. Biochem Biophys Res Commun 308:492–496. doi:10.1016/S0006-291X(03)01421-9
Schuppe-Koistinen I, Moldéus P, Bergman T, Cotgreave IA (1994) S-thiolation of human endothelial cell glyceraldehyde-3-phosphate dehydrogenase after hydrogen peroxide treatment. Eur J Biochem 221:1033–1037
Ravichandran V, Seres T, Moriguchi T (1994) S-thiolation of glyceraldehyde-3-phosphate dehydrogenase induced by the phagocytosis-associated respiratory burst in blood monocytes. J Biol Chem 269:25010–25015
Shenton D, Grant CM (2003) Protein S-thiolation targets glycolysis and protein synthesis in response to oxidative stress in the yeast Saccharomyces cerevisiae. Biochem J 374:513–519. doi:10.1042/BJ20030414
Grant CM, Quinn KA, Dawes IW (1999) Differential protein S-thiolation of glyceraldehyde-3-phosphate dehydrogenase isoenzymes influences sensitivity to oxidative stress. Mol Cell Biol 19:2650–2656
Nakajima H, Amano W, Kubo T et al (2009) Glyceraldehyde-3-phosphate dehydrogenase aggregate formation participates in oxidative stress-induced cell death. J Biol Chem 284:34331–34341. doi:10.1074/jbc.M109.027698
Butterfield DA, Hardas SS, Lange MLB (2010) Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer’s disease: many pathways to neurodegeneration. J Alzheimers Dis 20:369–393. doi:10.3233/JAD-2010-1375
Godon C, Lagniel G, Lee J et al (1998) The H2O2 stimulon in Saccharomyces cerevisiae. J Biol Chem 273:22480–22489
Ralser M, Wamelink MM, Kowald A et al (2007) Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. J Biol 6:10. doi:10.1186/jbiol61
Ralser M, Heeren G, Breitenbach M et al (2006) Triose phosphate isomerase deficiency is caused by altered dimerization – not catalytic inactivity – of the mutant enzymes. PLoS One 1:e30. doi:10.1371/journal.pone.0000030
Clower CV, Chatterjee D, Wang Z et al (2010) The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism. Proc Natl Acad Sci U S A 107:1894–1899. doi:10.1073/pnas.0914845107
Noguchi T, Yamada K, Inoue H et al (1987) The L- and R-type isozymes of rat pyruvate kinase are produced from a single gene by use of different promoters. J Biol Chem 262:14366–14371
Noguchi T, Inoue H, Tanaka T (1986) The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem 261:13807–13812
David CJ, Chen M, Assanah M et al (2010) HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 463:364–368. doi:10.1038/nature08697
Imamura K, Tanaka T (1972) Multimolecular forms of pyruvate kinase from rat and other mammalian tissues. I. Electrophoretic studies. J Biochem 71:1043–1051
Mazurek S (2011) Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol 43:969–980. doi:10.1016/j.biocel.2010.02.005
Keller KE, Doctor ZM, Dwyer ZW, Lee Y-S (2014) SAICAR induces protein kinase activity of PKM2 that is necessary for sustained proliferative signaling of cancer cells. Mol Cell 53:700–709. doi:10.1016/j.molcel.2014.02.015
Keller KE, Tan IS, Lee Y-S (2012) SAICAR stimulates pyruvate kinase isoform M2 and promotes cancer cell survival in glucose-limited conditions. Science 338:1069–1072. doi:10.1126/science.1224409
Chaneton B, Hillmann P, Zheng L et al (2013) Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature 491:458–462. doi:10.1038/nature11540
Ashizawa K, Willingham MC, Liang CM, Cheng SY (1991) In vivo regulation of monomer-tetramer conversion of pyruvate kinase subtype M2 by glucose is mediated via fructose 1,6-bisphosphate. J Biol Chem 266:16842–16846
Ikeda Y, Noguchi T (1998) Allosteric regulation of pyruvate kinase M2 isozyme involves a cysteine residue in the intersubunit contact. J Biol Chem 273:12227–12233
Anastasiou D, Yu Y, Israelsen WJ et al (2012) Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol 8:839–847. doi:10.1038/nchembio.1060
Christofk HR, Vander Heiden MG, Wu N et al (2008) Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature 452:181–186. doi:10.1038/nature06667
Lv L, Xu Y-P, Zhao D et al (2013) Mitogenic and oncogenic stimulation of K433 acetylation promotes PKM2 protein kinase activity and nuclear localization. Mol Cell 52:340–352. doi:10.1016/j.molcel.2013.09.004
Hitosugi T, Kang S, Vander Heiden MG et al (2009) Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal 2:ra73. doi:10.1126/scisignal.2000431
Gao J, Aksoy BA, Dogrusoz U et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:pl1–pl1. doi:10.1126/scisignal.2004088
Gao X, Wang H, Yang JJ et al (2012) Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell 45:598–609. doi:10.1016/j.molcel.2012.01.001
Israelsen WJ, Dayton TL, Davidson SM et al (2013) PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell 155:397–409. doi:10.1016/j.cell.2013.09.025
Yang W, Xia Y, Hawke D et al (2012) PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell 150:685–696. doi:10.1016/j.cell.2012.07.018
Christofk HR, Vander Heiden MG, Harris MH et al (2008) The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452:230–233. doi:10.1038/nature06734
McKnight SL (2014) Please keep me 2uned to PKM2. Mol Cell 53:683–684. doi:10.1016/j.molcel.2014.02.022
Yang W, Xia Y, Ji H et al (2011) Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature 480:118–122. doi:10.1038/nature10598
Anastasiou D, Poulogiannis G, Asara JM et al (2011) Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334:1278–1283. doi:10.1126/science.1211485
Maeba P, Sanwal BD (1968) The regulation of pyruvate kinase of Escherichia coli by fructose diphosphate and adenylic acid. J Biol Chem 243:448–450
McDonagh B, Ogueta S, Lasarte G, Padilla CA (2009) Shotgun redox proteomics identifies specifically modified cysteines in key metabolic enzymes under oxidative stress in Saccharomyces cerevisiae. J Proteomics 72:677–689. doi:10.1016/j.jprot.2009.01.023
Grüning N-M, Rinnerthaler M, Bluemlein K et al (2011) Pyruvate kinase triggers a metabolic feedback loop that controls redox metabolism in respiring cells. Cell Metab 14:415–427. doi:10.1016/j.cmet.2011.06.017
Grüning N-M, Du D, Keller MA et al (2014) Inhibition of triosephosphate isomerase by phosphoenolpyruvate in the feedback-regulation of glycolysis. Open Biol 4:130232. doi:10.1098/rsob.130232
Luo W, Hu H, Chang R et al (2011) Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145:732–744. doi:10.1016/j.cell.2011.03.054
Luo W, Semenza GL (2011) Pyruvate kinase M2 regulates glucose metabolism by functioning as a coactivator for hypoxia-inducible factor 1 in cancer cells. Oncotarget 2:551–556
Snell K, Natsumeda Y, Eble JN et al (1988) Enzymic imbalance in serine metabolism in human colon carcinoma and rat sarcoma. Br J Cancer 57:87–90
Snell K (1986) The duality of pathways for serine biosynthesis is a fallacy. TIBS 11(6):241–243
Mullarky E, Mattaini KR, Vander Heiden MG et al (2011) PHGDH amplification and altered glucose metabolism in human melanoma. Pigment Cell Melanoma Res 24:1112–1115. doi:10.1111/j.1755-148X.2011.00919.x
Barker GA, Ellory JC (1990) The identification of neutral amino acid transport systems. Exp Physiol 75:3–26
Palacín M, Estévez R, Bertran J, Zorzano A (1998) Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev 78:969–1054
Possemato R, Marks KM, Shaul YD et al (2011) Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476:346–350. doi:10.1038/nature10350
Locasale JW, Grassian AR, Melman T et al (2011) Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet 43:869–874. doi:10.1038/ng.890
Hitosugi T, Zhou L, Elf S et al (2012) Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth. Cancer Cell 22:585–600. doi:10.1016/j.ccr.2012.09.020
Futerman AH, Riezman H (2005) The ins and outs of sphingolipid synthesis. Trends Cell Biol 15:312–318. doi:10.1016/j.tcb.2005.04.006
Kuge O, Hasegawa K, Saito K, Nishijima M (1998) Control of phosphatidylserine biosynthesis through phosphatidylserine-mediated inhibition of phosphatidylserine synthase I in Chinese hamster ovary cells. Proc Natl Acad Sci 95:4199–4203
de Koning TJ, Snell K, Duran M et al (2003) L-serine in disease and development. Biochem J 371:653–661. doi:10.1042/BJ20021785
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033. doi:10.1126/science.1160809
Acknowledgments
We would like to thank Gina M. DeNicola, Jared L. Johnson, and Costas A. Lyssiotis for helpful discussions and comments on the manuscript. This work was supported by National Institutes of Health grants to L.C.C.: R01 GM041890, P01 CA117969, P01 CA120964.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this paper
Cite this paper
Mullarky, E., Cantley, L.C. (2015). Diverting Glycolysis to Combat Oxidative Stress. In: Nakao, K., Minato, N., Uemoto, S. (eds) Innovative Medicine. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55651-0_1
Download citation
DOI: https://doi.org/10.1007/978-4-431-55651-0_1
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55650-3
Online ISBN: 978-4-431-55651-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)