Abstract
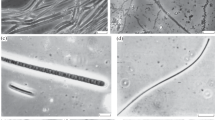
Colonization stages and early development of a matforming microbenthic community were studied during a 1-year period in a newly constructed hypersaline solar pond situated in the Dead Sea area. The pond had an inversely stratified water column consisting of a cool convective epilimnion of diluted Dead Sea water, a thermocline, and non-convective hot Dead Sea water at the bottom. The sharp halocline was maintained by adding fresh water to the upper water mass. Unicellular cyanobacteria and diatoms were the pioneer colonizers of the pond slopes. Metazoan grazers were absent, eliminated by the unique chemical composition of the Dead Sea water. Chlorophyll-a concentrations, reflecting photosynthetically active biomass, were highest within the shallow epilimnetic zone of the littoral (reaching 278 mg/m2), and declined abruptly in the gradient zone. Both temperature and salinity appeared to be major limiting factors in this environment. Cyanobacteria were more resistant to high temperatures, attaining optimal growth up to approximately 48 °C. Within optimal temperature limits, microbenthos development was best in the lower salinities of 50–70 g/1. Salinities of 100–150 g/1 seem to be an upper limit for this community. Chlorophyll-a decline at the onset of the gradient zone, where favorable temperatures and salinities prevailed, is attributed to pronounced salinity fluctuations occurring at this level. At the end of the first year, the young stromatolitic mat was several millimeters thick and well consolidated due to gelatinous polysaccharides excreted by cyanobacteria.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Adin A, Dor I (1982) Maintaining transparency in electricity generating solar ponds. Scientific report No. 3 to Solmat Systems. Hebrew Univ, Jerusalem, 278 pp (in Hebrew)
Adin A, Dor I, Sela S (1981) Removal of turbidity from electricity generating solar ponds. Scientific report No. 2 to Solmat Systems. Hebrew Univ, Jerusalem, 192 pp (in Hebrew)
Burne RV, Moore LS (1987) Microbialites: organosedimentary deposits of benthic microbial communities. Palaios 2:241–254
Cohen Y (1984) The Solar Lake cyanobacterial mats: strategies of photosynthetic life under sulfide. In: Cohen Y, Castenholz RW, Halvorson HO (eds) Microbial mats: stromatolites (MBL lectures in biology 3). Liss, New York, pp133–148
Cohen Y, Rosenberg E (eds) (1989) Microbial mats: physiological ecology of benthic microbial communities. Am Microbiol, Washington, 494 pp
CohenY, Castenholz RW, Halvorson HO (eds) (1984) Microbial mats: stromatolites (MBL lectures in biology 3). Liss, New York, XVIII + 498 pp
Dor I (1983) Research on algae of solar ponds. Report No. 1 to Solmat Systems. Hebrew Univ, Jerusalem, 46 pp
Dor I (1984 a) Epiphytic blue-green algae (cyanobacteria) of the Sinai Mangal: considerations on vertical zonation and morphological adaption. In: Por FD, Dor I (eds) Hydrobiology of the Mangal. Junk, The Hague, pp 35–54
Dor I (1984 b) Algal research in solar ponds. Scientific report No. 2 to Solmat Systems. Hebrew Univ, Jerusalem, 46 pp (in Hebrew)
Dorl, Ehrlich A (1987) The effect of salinity and temperature gradients on the distribution of littoral microalgae in experimental solar ponds, Dead Sea area, Israel. P.S.Z.N.I. Mar Ecol 8(3):193–205
Dorl, Hornoff M (1985a) Salinity-temperature relations and morphotypes of a mixed population of coccoid cyanobacteria from a hot, hypersaline pond in Israel. P.S.Z.N.I. Mar Ecol 6(1): 13–25
Dor I, Hornoff M (1985 b) Studies on Aphanothece halophyt ica Fremy from a solar pond: comparison of two isolates on the basis of cell polymorphism and growth response to salinity, temperature and light conditions. Bot Mar 28:389–398
Dor I, Paz N (1989) Temporal and spatial distribution of mat microalgae in the experimental solar ponds, Dead Sea area, Israel. In: Cohen Y, Rosenberg E (eds) Microbial mats: physiological ecology of benthic microbial communities. Am Soc Microbiol, Washington. pp 114–122
Friedman GM. Krumbein WE (eds) (1985) Hypersaline ecosystems: the Gavish Sabkha (Ecological Studies 53). Springer, Berlin Heidelberg New York. x 4– 484 pp
Gavrieli I (1987) The source of the halite bodies in the southern Dead Sea. Report No. GSI/11/87. M Sc Thesis, Hebrew Univ, Jerusalem, 123 pp (in Hebrew)
Geitler L (1932) Cyanophyceae. Kryptogamenflora von Deutschland, Österreich und der Schweiz, 14–1. Akademie, Leipzig, 1196 pp
Gerdes G. Krumbein WE. Holtkamp E (1985) Salinity and water activity related zonation of microbial communities and potential stromatolites of the Gavish Sabkha. In: Friedman GM, Krumbein WE (eds) Hypersaline ecosystems, the Gavish Sabkha. Springer, Berlin Heidelberg New York, pp 238–266
Glaessner MF (1983) The emergence of Metazoa in the early history of life. In: Nagy B, Weber R, Guerrero JC, Schidlowski M (eds) Developments and interactions of the Precambrian atmosphere, lithosphere and biosphere. Elsevier, Amsterdam, pp 319–333
Golubic S (1980) Halophily and halotolerance in cyanophytes. Orig Life 10:169–183
Javor B (1989) Hypersaline environments. Springer, Berlin Heidelberg New York, 328 pp
Kirkland DW, Bradbury JP, Dean WE (1983) The heliothermic lake -a direct method of collecting and storing solar energy. Arch Hydrobiol Suppl 65 (1): 1–60
Krumbein WE, Cohen Y, Shilo M (1977) Solar Lake (Sinai.) 4. Stromatolitic cyanobacterial mats. Limnol Oceanogr 22:635–656
Lazar B, Javor B, Erez J (1984) Total alkalinity in marinederived brines and pore waters associated with microbial mats. In: Cohen Y, Castenholz RW, Halverson HO (eds) Microbial mats: stromatolites. Liss, New York, pp 84–93
Miller RL, Kamykowski DL (1986) Short-term photosynthetic responses in the diatom Nitzschia americana to a simulated salinity environment. J Plankton Res 8(2):305–315
Nissenbaum A (1975) The microbiology and biogeochemis-try of the Dead Sea. Microbial Ecol 2:139–161
Paz N (1983) Algal activity in solar ponds. M Sc Thesis, Hebrew Univ, Jerusalem, 149 pp (in Hebrew)
Por FD (1980) A classification of hypersaline waters, based on trophic criteria. P.S.Z.N.I. Mar Ecol 1(2): 121–131
Reed RH, Ward SRC, Richardson DI, Moore DJ, Stewart WDP (1985) Multiphasic osmotic adjustment in a eury-haline cyanobacterium. FEMS Microbiol Lett 28: 225– 229
Schopf JW (ed) (1983) Earth’s earliest biosphere: its origin and evolution. Univ Press, Princeton, xxv + 543 pp
Tabor H (1980) Using solar ponds to store power from the sun. Impact Sci Soc 30(4):319–328
Tabor H (1981) Solar ponds. Solar Energy 27(3): 181–194
Tailing JF (1969) General outline of spectrophotometric methods. In: Vollenweider (ed) A manual on methods for measuring primary productivity in aquatic environments (IBP Handbook 12). Blackwell, Oxford, pp 22–25
Trüper HG, Galinski EA (1989) Compatible solutes in halophilic phototrophic procaryotes. In: Cohen Y, Rosenberg E (eds) Microbial mats: physiological ecology of benthic microbial communities. Am Soc Microbiol. Washington, pp 342–348
Walter MR (1983) Archean stromatolites: evidence of the earth’s earliest benthos. In: Schopf (ed) Earth’s earliest biosphere: its origin and evolution. Univ Press, Princeton, pp 187–213
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1992 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Dor, I., Carl, N., Schidlowski, M. (1992). Experimental Hypersaline Ponds as Model Environments for Stromatolite Formation 1. Microbenthos Composition and Biomass Accumulation. In: Schidlowski, M., Golubic, S., Kimberley, M.M., McKirdy, D.M., Trudinger, P.A. (eds) Early Organic Evolution. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-76884-2_39
Download citation
DOI: https://doi.org/10.1007/978-3-642-76884-2_39
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-76886-6
Online ISBN: 978-3-642-76884-2
eBook Packages: Springer Book Archive