Abstract
Stable carbon isotopes in tree-rings are not only useful to derive climatic information of the past. Based on the isotope fractionations during uptake and fixation of CO2, physiological information can be retrieved, namely the ratio of assimilation to stomatal conductance, which is termed the intrinsic water-use efficiency (iWUE). This crucial plant physiological trait varies among species and environments and is characteristic of how much water is lost from leaves for a certain carbon gain. iWUE is of great importance at the scale of individual plants because it can determine plant performance and survival. iWUE also contributes how closely canopy- or ecosystem-scale carbon and water fluxes are coupled or divergent, which has implications for understanding biogeochemical cycling. Carbon isotopes in tree-rings can be used to estimate how iWUE of trees has changed in the past, e.g. due to increasing CO2, nitrogen or other factors. Accordingly, many applications have explored this tool for various forest ecosystems across the globe, often reporting a strong increase in iWUE over the twentieth century. Explicit comparisons of tree-ring iWUE to growth-data obtained from the same rings can help distinguish among strategies plants employ under various environmental impacts, like increasing CO2, light limitation, drought or too much water. In this chapter, we describe the theory behind iWUE, show some limitations of the method, give examples of the combined application of iWUE and tree-ring width, discuss photosynthetic limitations of iWUE and finally show how the method has been applied in large-scale tree-ring networks.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Introduction
The development of the Farquhar-model of carbon isotope fractionation during photosynthesis was a milestone in the application of stable isotopes in ecology and many other fields (Farquhar et al. 1982). This model enabled a straightforward interpretation of carbon isotope values of plant organic matter in terms of physiology. One of the primary predictands of the Farquhar model is the internal CO2-concentration inside the leaf (ci). Soon it was realized that this predictand can be directly linked to the so-called intrinsic water-use efficiency (iWUE), the ratio between assimilation (A) and stomatal conductance for water vapour (g) (Ehleringer and Cerling 1995; Farquhar et al. 1989) (see Sect. 17.2 for more details). The regulation of stomatal opening is one of the most intricate and essential functions of terrestrial plants, as water-limited systems demand that stomatal behavior simultaneously constrain water loss while assuring sufficient carbon gain for survival. This makes iWUE such a useful property to know, although the actual water-use efficiency (WUE), defined as the ratio of transpiration to assimilation, may even be more relevant in determining how plants respond to dry conditions. The analysis of carbon isotope values of organic matter is nowadays an efficient method to determine iWUE that integrates minute-to-minute signals in leaves over the days to months it may take to synthesize plant tissues. The isotopic composition can be determined efficiently via on-line coupling of elemental analysers to isotope-ratio mass-spectrometers (see Chap. 7), which has allowed for scientists to produce much larger data sets compared to studies taking place 20 years ago.
Given the advancements in isotopic theory and technical ability, it is not surprising that the Farquhar-model has found increasingly widespread application in tree-ring studies e.g. (Marshall and Monserud 1996; Penuelas et al. 2011; Waterhouse et al. 2004). In turn, many investigators have realized that the doors have been flung open to reveal retrospective insights on how physiological processes have shifted in response to myriad changing environmental conditions. Particularly important avenues of research for projecting carbon-climate-vegetation feedbacks within the biosphere have addressed how forests have responded to ongoing climate change and increasing CO2 in the atmosphere (Saurer et al. 2014, Voelker et al. 2016). Although numerous studies of how plants have responded to CO2, temperature, drought and other factors have been carried out in greenhouses or growth chambers, such studies of small plants may not be representative of how adult trees in natural environments may have responded. Where tree longevity and size have made experimentation extraordinarily difficult, the use of tree-ring stable carbon isotopes can provide a more realistic view by studying trees in their natural habitat and over their entire life-cycle. Intriguingly, information on iWUE can be retrieved for times when CO2-concentration was different, for instance on the pre-industrial level of 280 ppm compared to current levels that exceed 400 ppm. We can thus obtain information on the ratio between A and g of trees living at times when scientific inquiry into plant function was in its infancy and when leaf gas-exchange equipment did not even exist. Stable carbon isotopes of tree-ring cellulose can therefore provide accurately and absolutely dated archives of annual and intra-annual past plant physiological responses to climate, CO2 and other environmental drivers that are unavailable from experimental methods and other paleoecological data sources.
Despite the power of pairing carbon isotope measurements and theory with tree-rings, some limitations should also be mentioned (Sect. 17.3). As iWUE only resolves the ratio of A to g, it often remains elusive, which of the two changed and to what degree. An increase in iWUE, for instance, can theoretically be caused by higher A or by lower g or a combination of the two. Furthermore, it should always be considered that iWUE is a different metric than the actual WUE (see Sect. 17.3 for details). Nevertheless, independent data confirm the usefulness of δ13C-derived iWUE-estimates from tree rings. Accordingly, iWUE-time series from multiple sites across the globe have provided numerous and invaluable insights into tree responses to global climate change. Increases in iWUE have been documented by essentially all studies spanning multiple decades of tree-ring isotope data and have occurred within the last ca. 100 years. However, the rate of change of increase in iWUE is quite variable and the reason for this range of responses not well understood. To provide additional insights, some studies have combined tree-ring derived iWUE with growth data originating from the same tree-rings. This can help the interpretation of growth trajectories by adding a physiological perspective, for instance in studies of drought-related decline (Sect. 17.4). While most studies focused on stomatal limitations of iWUE, photosynthetic limitation may be an under-explored topic (Sect. 17.5). Finally, due to the construction of large networks of sites with tree-ring isotope data, it recently became feasible to study spatial patterns of iWUE on regional to continental scales (Sect. 17.6). Hence, with these examples, the breadth and success of iWUE-reconstructions using tree-ring isotopes is on full display and portends many novel findings in the future.
2 Model and Scenarios
The potential to derive physiological information from δ13C of plant material (δ13Cplant) is strongly based on the Farquhar-model (1982), which in its simplest form is given as:
where δ13Catm is the carbon isotope ratio of atmospheric CO2, a (4.4‰) is the fractionation associated with the diffusion of CO2 through the stomata, b (27‰) the fractionation resulting from enzymatic C fixation by RubisCO, and ci/ca is the ratio of leaf internal to ambient CO2-concentrations. This mechanistic model for C3-plants was experimentally verified in many studies (Evans et al. 1986) and predicts a depletion in the isotope ratio in the plant compared to the isotope ratio in the atmosphere, but to a varying degree depending on ci/ca. Equation (17.1) can be applied directly to tree-rings by using the measured tree-ring isotope value of a specific year as δ13Cplant, provided the corresponding value for δ13Catm for the same year is used. The δ13Catm-values have declined over the past 150 years due to fossil CO2 emissions and are known either from atmospheric measurements or derived from ice-core studies (Leuenberger 2007). A modified equation is sometimes used in biological studies, based on carbon isotope discrimination (∆), which approximates the difference between δ13Catm and δ13Cplant, thus a positive number:
These equations can be solved for ci/ca. In the following, we explain how the intrinsic water-use efficiency iWUE is derived from this information, which is the ratio of net photosynthesis (A) to conductance for water vapor (gH2O) (Ehleringer and Cerling 1995):
expressed in units of μmol mol−1. Using the equation for net photosynthesis
with gCO2 as the conductance for CO2, and considering
we obtain the following relationship
Finally, using ci/ca derived from Eq. (17.1), we find:
Such derived iWUE has been widely employed in tree-ring isotopic studies.
A and gH2O and thus ci depend on various environmental drivers (light, CO2, VPD, etc.) and are dependent on species and site conditions. To group different tree physiological responses, a useful heuristic has classified three primary leaf gas-exchange scenarios (Saurer et al. 2004): In response to changing CO2 and other environmental variability over time, there can be trees that (1) tend to keep ci constant representing a homoeostatic gas-exchange regulation (Marshall and Monserud 1996), (2) trees that keep ci/ca constant like a set-point (Ehleringer and Cerling 1995), or (3) trees that keep ca-ci constant, which is the equivalent of no increase in iWUE. Over large gradients in ca, however, meta-analysis of empirical data indicates there is a shift between scenarios (Voelker et al. 2016). Equations (17.1 and 17.7) shown here are easily applicable due to their simple form, but additional fractionation effects occur during and after photosynthesis that could in principle also be included. Such effects are, for instance, due to the mesophyll conductance and other diffusive limitations within the leaf as well as photorespiration (discussed in Chap. 9), post-photosynthetic fractionations during biochemical reactions and phloem transport (Chap. 13) as well as effects related to timing of wood formation, use of stored carbohydrates and subsequent mixing of carbon pools of different age (Chap. 15).
3 Limitations and Verifications
While iWUE has proven useful in numerous studies, one needs to be aware of some limitations of this metric. The actual WUE is a closely related, but still different concept, which is calculated as the ratios of net photosynthesis (A) to transpiration (E) (rather than A to gH2O only):
with E defined as:
whereby ei and ea are the vapor pressures in the leaf cellular air space and ambient air, respectively. Such WUE may be ecologically more relevant than iWUE because it is based on the water fluxes and depends directly on VPD, which has been shown to be important in amplifying recent warming trends (Breshears et al. 2013; Szejner et al. 2019). Furthermore, WUE can be calculated over different periods, for instance, as the ratio of carbon uptake to water loss at the plant level over a growing season or plant life, which is also influenced by respiratory losses. It has been shown that different concepts of WUE, like intrinsic and actual WUE, are sometimes poorly correlated (Seibt et al. 2008). WUE can also be considered at a larger scale: at the ecosystem-level, where it is defined as gross primary production (GPP) relative to evapotranspiration (ET). In our opinion, it is of great importance to be aware of the different scales at which WUE can be calculated and be cognizant that one is not compared directly with another without acknowledging how they may differ. It should be quite obvious that plant-level and, for instance, ecosystem level WUE are not the same and therefore should also not be expected to respond similarly to environmental variability. Nonetheless, comparisons of WUE-estimates obtained by different techniques may yield new insights on leaf, plant and ecosystem functioning.
As an example where different techniques resulted in comparable WUE estimates, a study in a Quercus petraea forest indicated that seasonal iWUE data obtained via a process-based physiological model matched well with iWUE derived from intra-annual δ13C of tree-rings (Michelot et al. 2011). The authors concluded that latewood may be a good proxy for assessing seasonal variations of the ratio of assimilation to stomatal conductance, despite some delay in organic matter deposition in the ring. In contrast, WUE at the ecosystem level determined as GPP/ET at eddy-covariance-based ecosystem flux tower monitoring sites across North America showed that WUE at the ecosystem scale has been increasing much faster than that recorded by tree-ring isotope-based iWUE (Guerrieri et al. 2019). The authors speculated that reasons for this discrepancy could be different time-scales of the two approaches or fluxes not accounted for, like non-transpirational water fluxes and contribution from understory vegetation. In a study using satellite-based NPP estimates, a relationship between tree-ring δ13C values of an Eastern US network of sites and NPP was found, rather than with WUE (Levesque et al. 2019). Finally, at a site in California, Keen (2019) showed that intra-annual tree-ring derived iWUE responded positively to VPD, whereas ecosystem-level WUE responded negatively to VPD, and this opposing VPD-response drove a negative response between iWUE and WUE during a range of wet to historically severe drought conditions. Together, these studies demonstrate that tree-ring carbon isotopes can contain valuable information on large-scale fluxes, but that more studies are needed to determine under what conditions iWUE is related to ecosystem-scale WUE (Seibt et al. 2008).
A complication of interpreting tree growth patterns and iWUE-estimation that needs careful consideration are the effects of age, tree size or height (Brienen et al. 2017; McDowell et al. 2011). Dendrochronologists have conventionally used empirical detrending methods to remove age-related growth patterns from ring-width data prior to assessing climate-sensitivity of tree growth. In recent decades it has become popular among ecologists and ecophysiologists to convert tree-ring data to basal area increment (BAI) or BAI/basal area for a given year, often putatively as a means to avoid the need to detrend ring-width data that decline with tree size and age. However, the often-stated or implied contention that these variables overcome the need for detrending is a misconception. For example, BAI tends to increase with tree size, but trees with initially greater BAI tend to have steeper positive relationships between BAI and tree age (Voelker et al. 2008). Likewise, BAI/basal area has a negative trend with age that is particularly steep when trees are young and BAI/basal area is nearer to one. Hence, it should be obvious that use of BAI or BAI/basal area may include biases in tree growth trends and should not be compared when groups of trees differ in age or size. Overall, BAI or BAI/basal area methods may not be an improvement over conventional detrending methods for comparison to stable isotopes in the same tree rings, particularly for studies of trees that are young (i.e., <100 years in age).
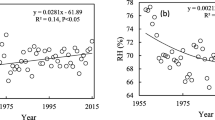
The carbon isotopes of tree-rings can change as trees grow, and depending somewhat on species and stand density, foliage is displayed at further distances from soil water sources as they get taller and form larger branches (McDowell et al. 2011). This change in stature results in a greater hydrostatic gradient in water potential and higher hydraulic resistance that impacts canopy-level stomatal conductance and thus iWUE. Such long-term trends owing to changes in tree stature can be mistakenly attributed to climate and/or CO2, so care should be taken to minimize the potential for this bias by considering it in assessments of carbon isotope responses to long-term environmental change (Brienen et al. 2017). If neglected, this bias could result in an over-estimation of how CO2 modifies leaf gas exchange. Several studies have, however, highlighted that δ13C mainly has age-related trends in the first few decades, forming a so-called juvenile trend, but not later on (Gagen et al. 2007; McCarroll and Loader 2004). This problem can be avoided by simply not using the juvenile phase or by applying appropriate corrections if known for a specific species or site (Vadeboncoeur et al. 2020). The age-trend can well be tested for trees growing in pre-industrial times where there are no strong variations in CO2-concentration by aligning them according their cambial age. This was done in a recent study with coastal redwood trees where indeed the age-trend was strongest in the first few decades of their life, but extended several centuries before finally levelling off (Voelker et al. 2018) (Fig. 17.1). These trees are, however, almost 100 m tall and take exceptionally long to reach this height, showing that there is no universal juvenile phase that is applicable across species and forest types.
Examples of inter-annual iWUE, sampled for two intra-annual ring divisions (latewood and middlewood), from seven coastal redwoods (Sequoia sempervirens) growing in northern California, USA (after Voelker et al. (2018)). Tree age data were corrected to ground level from tree cross-section sampling height and pith dates were estimated using conventional techniques. To minimize the potential impact of atmospheric CO2 concentrations or 13CO2, no data from years later than 1880 were used. Finally, tree-level variation was minimized by iteratively fitting negative exponential curves and correcting each data point with the tree-level mean residual. There were no significant changes to the relationship after three iterations. Note that middlewood was defined as the central 60% of each ring while the latewood occupied the last 25% and the first 15% of each ring was discarded
Some studies have tried to exclude age-effects by comparing different age cohorts, i.e. comparing iWUE of young trees with mature trees during the same time period (Bert et al. 1997; Brienen et al. 2017; Marshall and Monserud 1996). These studies have not addressed a potential sampling bias, whereby mature trees are the survivors of decades to centuries of mortality processes and also may not reflect the overall stand structure and history (Brienen et al. 2017). Microclimatic conditions are also different for saplings near the ground as compared to larger trees, e.g. regarding light and VPD, which affects the relationship between δ13C and growth (Fardusi et al. 2016). In other studies, stand structure has been shown to affect δ13C-trends of understory beech and spruce trees, emphasizing the effects of competition and light (Klesse et al. 2018) as well as in overstory ponderosa pine and grand fir trees (Voelker et al. 2019a) due to increasing competition for water in the absence of wildfire. Clearly, more research is needed to reliably separate the effects of CO2, climate, age and tree height. Nevertheless, the strong increases in iWUE as a result of the atmospheric CO2-increase has been found to be widespread and thereby clearly cannot be an artefact (Guerrieri et al. 2019).
4 Combination of Growth and iWUE: The Case of Drought
The combination of conventional dendrochronological analyses of growth and stable isotope methods can be very powerful. With a multi-proxy approach, the particular strength of tree rings is taken full advantage of as all the measurable parameters originate from the same absolutely dated rings, but contain different environmental information. Tree ring widths or basal area increments provide cumulative growth information that is related to the complex interplay of environmental conditions and limiting factors to xylogenesis during the growing season, whereas stable isotopes measured in the cellulose of these tree rings are rather recording the physiological conditions at the canopy level. Stable isotopes are also influenced by seasonal timing of wood formation as periods of extreme growth limitation, e.g. drought, may not be reflected in the isotope signal of the tree ring when xylogenesis is halted (Sarris et al. 2013). Furthermore, drought legacy effects may differ between tree-ring width and stable isotopes as the recovery after an extreme event may be different for the two parameters (Szejner et al. 2019). Hence, stable isotopes can help decipher causes of growth variations more clearly in many cases. Tree growth and iWUE may or may not show similar trends and may also display positive or negative correlations. With careful consideration of biological trends due to tree age and size, the combined analysis of tree growth and iWUE or carbon isotope discrimination can help to decipher what relevant environmental mechanisms may be impacting trees by affecting leaf gas exchange, growth, or both factors together (Brienen et al. 2021; Sun et al. 2018; Voelker et al. 2014). This multi-proxy approach may be particularly relevant for better understanding tree responses to climate warming and various aspects of drought (Levesque et al. 2014). Indeed, the area of land classified as very dry has more than doubled globally in the last 50 years and accordingly drought conditions are affecting many forest ecosystems, resulting in reduced growth and increased stress and tree mortality (Allen et al. 2010). For example, in areas such as the Central Mediterranean, Central and Western Europe, California and much of the Western United States and elsewhere, warmer temperatures have amplified droughts (Dai 2013). California and the west US coast in particular has undergone recent extremes (Keen et al. 2022) that are expected to become more severe in the future based on climate model predictions (Wang et al. 2017; Yoon et al. 2015). These projections are further supported by long tree-ring isotope chronologies that demonstrated greater hydroclimate variability during the warmer Medieval Climate Anomaly and the period of recent warming compared to the colder Little Ice Age (Voelker et al. 2018).
Considering projections of increased drought frequency and severity, there is a crucial need to understand mechanisms leading to drought-induced tree mortality, which may be provided by investigating how some trees, species and different forest ecosystems have responded to acute or chronic drought stress (Allen et al. 2010). Although drought-related physiological effects on trees have long been been studied, the significance of different mechanisms is still unclear (McDowell et al. 2008). When pests and pathogens do not act as the primary cause of tree mortality, the most important processes leading to death are hydraulic failure (desiccation) in extreme drought events and a reduction of the trees’ carbon storage through gradual depletion of carbohydrates (starvation) induced by protracted drought episodes. The mechanism of carbon starvation is likely related to water-use efficiency, as plants may reduce stomatal conductance strongly to avoid loss of water, and therefore this process may be elucidated using stable carbon isotopes. In such a situation, the plant is no longer capable of producing enough non-structural carbohydrates to maintain essential metabolic functions and stored non-structural carbohydrates are inaccessible due to compartmentalization and lack of enzymatic energy (Sala et al. 2010).
Studies of trees that survived or died during intense periods of drought have been particularly effective at applying tree-ring growth and stable carbon isotopes to highlight physiological factors that ostensibly predisposed trees to mortality. One of the first such investigations to do so used a rather narrow time window for assessment, but nonetheless found that trees that died during a severe drought had similar iWUE, but lower tree growth rates, compared to co-occurring trees that survived (McDowell et al. 2010). However, the surviving trees showed a strong climatic sensitivity of gas exchange (i.e. δ13C) in contrast to dying trees, which was attributed to dead trees having undergone chronic drought stress and carbon starvation prior to death. Other more recent studies have further refined this type of approach. For example, high mortality rates of Scots pine (Pinus sylvestris L.) in lower altitudes in inner-Alpine valleys such as the Valais (Switzerland) (Rigling et al. 2013) exemplify a region that was undergoing drought-induced forest decline. At one site, analyses of growth and stable carbon isotope ratios in tree rings over the twentieth century were combined with a 10-year irrigation experiment that doubled annual precipitation (Timofeeva et al. 2017). There was a strong growth increase and concurrent depletion of δ13C values for irrigated trees, indicating reduced iWUE. This demonstrated that progressive limitation of leaf gas-exchange by drought-induced stomatal closure was reversible when extra water was supplied. In the same stand, Scots pine trees that had recently died had more than 100 years of lower growth and higher iWUE derived from δ13C values compared with surviving trees. This indicates a conservative water-use strategy for trees that had died, which resulted in a lack of carbohydrates, reduction of the needle mass and long-term weakening. In contrast, a study with Norway Spruce (Picea abies L. Karst.) indicated a different cause for mortality, as dying trees grew significantly better and had higher iWUE in the earlier life phase than surviving trees (Hentschel et al. 2014). Similarly, it was found for Scots pine (Voltas et al. 2013) and for a Mediterranean oak species (Colangelo et al. 2017) that “fast growing” and “less efficient” individuals were more affected, whereas (Heres et al. 2014) observed that declining trees were less sensitive in iWUE than non‐declining trees. Such differences in tree response strategies to drought could be summarized in a conceptual diagram (Fig. 17.2).
Conceptual diagram for explaining death or survival of trees from the same stand based on their earlier physiology and growth patterns. The location of the black circle indicates the position of dead trees from the Scots pine site in Switzerland (Timofeeva et al. 2017), while the grey circle refers to Norway spruce trees from southern Norway (Hentschel et al. 2014). Surviving trees indicated as open circle are considered as the reference in both studies
The diagram shows differences between surviving and dead trees, where the surviving trees are considered as the reference. These two tree groups have differed in their past growth patterns and physiology prior to the actual, final decline phase. Trees following a conservative strategy are located in the upper, left-hand sector of the diagram, as was observed for Scots pine at the Swiss study site (Fig. 17.2). These trees are expected to be prone to carbon starvation rather than to hydraulic failure. It should be considered that carbon starvation may just mean a lack of carbohydrates and energy to maintain vital functions, although not a complete exhaustion of storage pools (Hartmann and Trumbore 2016). In contrast, for Norway spruce (Hentschel et al. 2014), trees that died later had higher growth and were not following a strict water-use strategy. Therefore, these trees were prone to hydraulic failure rather than carbon starvation. This less conservative water-use strategy falls in the lower, right-hand sector of the diagram (Fig. 17.2).
Absolute values of growth and iWUE can provide one continuum on which trees can be ordered that can provide valuable physiological interpretations (Fig. 17.2). Additional insights may be gained by assessing the sensitivity of growth or carbon isotopes to various metrics of meteorological drought or the coupling of growth to carbon isotope variation each provide additional windows on the relative degree of drought stress and how that response may have changed over time (Keen 2019, McDowell et al. 2010, Urrutia-Jalabert et al. 2015, Voelker et al. 2014, Voelker et al. 2019a). Overall, stable isotope analysis in combination with the study of growth patterns is therefore a promising approach for elucidating relevant physiological processes under drought, even more so when including oxygen isotope ratios as their changes are influenced by transpiration rate, but not photosynthesis (Gessler et al. 2018) (Chap. 10). This may ultimately result in improved predictions of forest ecosystem changes in the future.
5 Photosynthetic Limitations to iWUE
Most investigations of tree-ring carbon isotopes have highlighted how interannual variability in iWUE is driven by stomatal closure during drought and that this pattern is superimposed on how gradually rising CO2 has driven long-term changes in A. Since so few studies have demonstrated evidence for how A has formed the primary physiological constraint on interannual variability on tree-ring carbon isotopes and iWUE, a few examples warrant specific mention here because they may continue to lead to particularly novel insights. Two forest health studies on species located in the eastern United States have shown that iWUE has been strongly influenced by sulfur emissions and associated acidic deposition that increased during most of the twentieth century and then showed a reversal in trend near 1980 after US federal legislation and enforcement was increasingly implemented from 1963 through 1990 (Mathias and Thomas 2018; Thomas et al. 2013). The authors concluded that increasing A was an important component for explaining recent tree recovery. Other records where inter-annual variability in tree-ring isotopes were controlled by how A was modified by temperature and/or sunshine occurred in cold northerly regions such as Northern Norway and Sweden (Loader et al. 2013; Young et al. 2012). Alternatively, Voelker et al. (2019b) showed that similar constraints on A by temperature could be identified in temperate trees growing adjacent Lake Superior, which modulates near-lake air temperature regimes due to the large heat capacity of the lake reflecting previous winter conditions during spring and early summer. The last study was careful to utilize only “middlewood” formed during the early growing season, which contrasts with many other studies of drought stress that focus on whole rings or latewood. Hence, this emphasizes the need for future studies of tree-ring isotopes to carefully consider which intra-annual sampling scheme may be most appropriate where differences in the primary constraints on leaf carbon uptake may shift on a seasonal basis between photosynthetic rates and stomatal conductance (see also Chap. 15). Finally, Breinen et al. (2021) has shown that during understory phases of tree development, tree growth is often negatively correlated to carbon isotope discrimination but had neutral or weak positive correlations once the same trees were in canopy dominant positions, which implies that both the photosynthetic rates and growth of these trees were limited by irradiance when they were in the understory.
6 Large-Scale Patterns
Earth System Models and Dynamic Global Vegetation Models (DGVMs) are the workhorses to explore many large-scale terrestrial processes and climate-land biosphere interactions. These models are routinely used to project future changes in climate and Earth System processes under anthropogenic forcing in a detailed spatio-temporal context (Sitch et al. 2003). DGVMs represent the basic physiological processes responsible for plant growth via sets of interconnected equations such as those for photosynthesis, respiration, and stomatal conductance, and the dependence of these physiological processes on the environmental (e.g., atmospheric CO2 concentration, temperature, and water availability) conditions. A particular challenge for these models is to correctly capture and project changes at decadal-to-century timescales of utmost relevance for predicting short-term anthropogenic climate perturbations. For example, mechanisms of the contemporary terrestrial carbon sink are poorly understood which leads to large uncertainties in twenty-first century climate projections (Cox et al. 2000). The representation of many physiological mechanisms and plant-atmosphere interactions is still crudely or not at all implemented in these DGVMs, and appears to significantly contribute to uncertainties in predictions. Improving and validating the modeling of key processes with empirical data are thus particularly important.
Based on development of tree-ring isotopic networks over the past two decades (Saurer et al. 2014; Treydte et al. 2007), it has become feasible to link such tree-ring data to DGVMs on a large spatial and temporal scale. While not strictly leading to a validation of either model nor tree-ring data, this comparison can be profitable for both fields of scientific inquiry, as independent assessment of similar variables, like iWUE. Furthermore, DGVMs can also add more interpretation to a tree-ring network, as variables like gs and A can be obtained. In a recent study using a 35-site network across Europe with coniferous and deciduous species, changes in iWUE from 1901–2000 and the spatial distribution of these changes across Europe were investigated (Saurer et al. 2014). On average, iWUE increased in European forests by 28%, with clear spatial differences across the continent. Moreover, comparison of these data with iWUE simulations by a dynamic vegetation model (LPX-Bern 1.0) showed good agreement with spatial patterns and overall twentieth century trends in tree ring derived iWUE. Across the 27 conifer sites, tree-ring derived iWUE showed strong differences between sites grouped in three latitudinal bands, i.e. <45°N, 45–60°N, and >60°N (Fig. 17.3). The northermost sites showed the lowest iWUE and also the lowest increase over the twentieth century (60.3–70.3 μmol/mol from the first to last decade of twentieth century, i.e. an increase by 16.6%). They also showed a rather homogenous signal as reflected in relatively low variability among sites. In comparison, the most southern sites, from Mediterranean climate, showed the highest overall values, an increase of 23% over the twentieth century, and also were characterized by relatively high variability among sites, showing that different ecosystems react rather differently to increasing CO2 depending on local site factors. Sites from temperate climates, i.e. intermediate latitudes, showed intermediate overall iWUE, the strongest increase over the twentieth century by 31%, and further a notable increase in the variability over time. This patterns of iWUE-increase might reflect a generally strong, but variable sensitivity to increasing CO2, compared to where temperature (i.e. northern latitude sites) or precipitation (i.e. low latitude mediterrranean sites) have historically been strongly limiting to leaf gas-exchange and growth.
These analyses clearly demonstrate the usefulness of such networks of tree-ring data for better understanding physiological tree responses to climate and CO2 across different ecosystems. It might be further interesting to consider that net photosynthesis tends to play a larger role in determining growth and δ13C in more energy‐limited environments, such as boreal forests, than in drought‐prone environments. Under scenarios of increasing drought, this may imply that changes in the relative weight of energy and water limitations could be assessed through the combined use of δ13C and tree‐rings. Nevertheless, the interpretation of iWUE-trends is not straightforward and can be enhanced by DGVM-results as shown in another recent study (Frank et al. 2015). Here, increases in European forest transpiration were calculated over the twentieth century, although a general decrease in stomatal conductance was also inferred, which seems at first view to be contradictive. The increased model transpiration results were due to longer growing seasons, enhanced evaporative demand in a warmer climate, and increased leaf area, which in total were outweighing the effect of reduced conductance. These results suggest caution may need to be applied when interpreting iWUE-results physiologically from δ13C of tree-rings without additional information on seasonality of tree-ring growth, but also shows the enormous potential of combining tree-ring isotopes and DGVM- or other large-scale model outputs for yielding new insights on carbon and water cycling.
References
Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EH, Gonzalez P, Fensham R, Zhang Z, Castro J, Demidova N, Lim JH, Allard G, Running SW, Semerci A, Cobb N (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag 259:660–684
Bert D, Leavitt SW, Dupouey JL (1997) Variations of wood δ13C and water-use efficiency of Abies alba during the last century. Ecology 78:1588–1596
Breshears DD, Adams HD, Eamus D, McDowell NG, Law DJ, Will RE, Williams AP, Zou CB (2013) The critical amplifying role of increasing atmospheric moisture demand on tree mortality and associated regional die-off. Front Plant Sci 4
Brienen RJW, Gloor E, Clerici S, Newton R, Arppe L, Boom A, Bottrell S, Callaghan M, Heaton T, Helama S, Helle G, Leng MJ, Mielikainen K, Oinonen M, Timonen M (2017) Tree height strongly affects estimates of water-use efficiency responses to climate and CO2 using isotopes. Nat Commun 8
Brienen R, Helle G, Pons T, Boom A, Gloor M, Groenendijk P, Clerici S, Leng M, Jones C (2021) Paired analysis of tree ring width and carbon isotopes indicates when controls on tropical tree growth change from light to water limitations. Tree Physiol tpab142. https://doi.org/10.1093/treephys/tpab142
Colangelo M, Camarero JJ, Battipaglia G, Borghetti M, De Micco V, Gentilesca T, Ripullone F (2017) A multi-proxy assessment of dieback causes in a Mediterranean oak species. Tree Physiol 37:617–631
Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ (2000) Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408:184–187
Dai A (2013) Increasing drought under global warming in observations and models. Nat Clim Chang 3:52–58
Ehleringer JR, Cerling TE (1995) Atmospheric CO2 and the ratio of intercellular to ambient CO2 concentrations in plants. Tree Physiol 15:105–111
Evans JR, Sharkey TD, Berry JA, Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas-exchange to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol 13:281–292
Fardusi MJ, Ferrio JP, Comas C, Voltas J, de Dios VR, Serrano L (2016) Intra-specific association between carbon isotope composition and productivity in woody plants: a meta-analysis. Plant Sci 251:110–118
Farquhar GD, O’Leary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9:121–137
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Frank DC, Poulter B, Saurer M, Esper J, Helle G, Treydte KS, Zimmermann NE, Andreu L, Bednarz Z, Berninger F, Böttger T, D'Allessandro CD, Daux V, Filot M, Grabner M, Gutierrez E, Haupt M, Hilasvuori E, Jungner H, Kalela-Brundin M, Krapiec M, Leuenberger M, Loader NJ, Marah H, Masson-Delmotte V, Pazdur A, Pawelczyk S, Pierre M, Planells O, Pukiene R, Reynolds-Henne CE, Rinne KT, Saracino A, Sonninen E, Stievenard M, Switsur VR, Szczepanek M, Szychowska-Krapiec E, Todaro L, Waterhouse JS, Weigl M, Schleser GH (2015) Water use efficiency and transpiration across European forests during the Anthropocene. Nat Clim Chang 5:579
Gagen M, McCarroll D, Loader NJ, Robertson L, Jalkanen R, Anchukaitis KJ (2007) Exorcising the ‘segment length curse’: summer temperature reconstruction since AD 1640 using non-detrended stable carbon isotope ratios from pine trees in northern Finland. Holocene 17:435–446
Gessler A, Cailleret M, Joseph J, Schonbeck L, Schaub M, Lehmann M, Treydte K, Rigling A, Timofeeva G, Saurer M (2018) Drought induced tree mortality—a tree-ring isotope based conceptual model to assess mechanisms and predispositions. New Phytol 219:485–490
Guerrieri R, Belmecheri S, Ollinger SV, Asbjornsen H, Jennings K, Xiao J, Stocker BD, Martin M, Hollinger DY, Bracho-Garrillo R, Clark K, Dore S, Kolb T, Munger JW, Novick K, Richardson AD (2019) Disentangling the role of photosynthesis and stomatal conductance on rising forest water-use efficiency. Proc Natl Acad Sci USA 116:16909–16914
Hartmann H, Trumbore S (2016) Understanding the roles of nonstructural carbohydrates in forest trees—from what we can measure to what we want to know. New Phytol 211:386–403
Hentschel R, Rosner S, Kayler ZE, Andreassen K, Borja I, Solberg S, Tveito OE, Priesack E, Gessler A (2014) Norway spruce physiological and anatomical predisposition to dieback. For Ecol Manag 322:27–36
Heres AM, Voltas J, Lopez BC, Martinez-Vilalta J (2014) Drought-induced mortality selectively affects Scots pine trees that show limited intrinsic water-use efficiency responsiveness to raising atmospheric CO2. Funct Plant Biol 41:244–256
Keen RM (2019) Using tree-ring growth and stable isotopes to explore ponderosa pine ecophysiological responses to climate variability and the 2012–2015 California drought. Master’s thesis, Utah State University
Keen RM, Voelker SL, Wang S-YS, Bentz BJ, Goulden ML, Dangerfield CR, Reed CC, Hood SM, Csank AZ, Dawson TE, Merschel AG, Still CJ (2022) Changes in tree drought sensitivity provided early warning signal to the California drought and forest mortality event. Global Change Biol 28: 1119–1132
Klesse S, Weigt R, Treydte K, Saurer M, Schmid L, Siegwolf RTW, Frank DC (2018) Oxygen isotopes in tree rings are less sensitive to changes in tree size and relative canopy position than carbon isotopes. Plant Cell Environ 41:2899–2914
Leuenberger M (2007) To what extent can ice core data contribute to the understanding of plant ecological developments of the past? In: Dawson TE, Siegwolf RTW (eds) Stable isotopes as indicators of ecological change. Elsevier Academic Press, London, pp 211–233
Levesque M, Siegwolf R, Saurer M, Eilmann B, Rigling A (2014) Increased water-use efficiency does not lead to enhanced tree growth under xeric and mesic conditions. New Phytol 203:94–109
Levesque M, Andreu-Hayles L, Smith WK, Williams AP, Hobi ML, Allred BW, Pederson N (2019) Tree-ring isotopes capture interannual vegetation productivity dynamics at the biome scale. Nat Commun 10
Loader NJ, Young GHF, Grudd H, McCarroll D (2013) Stable carbon isotopes from Tornetrask, northern Sweden provide a millennial length reconstruction of summer sunshine and its relationship to Arctic circulation. Quat Sci Rev 62:97–113
Marshall JD, Monserud RA (1996) Homeostatic gas-exchange parameters inferred from 13C/12C in tree rings of conifers. Oecologia 105:13–21
Mathias JM, Thomas RB (2018) Disentangling the effects of acidic air pollution, atmospheric CO2, and climate change on recent growth of red spruce trees in the Central Appalachian Mountains. Glob Chang Biol 24:3938–3953
McCarroll D, Loader NJ (2004) Stable isotopes in tree rings. Quat Sci Rev 23:771–801
McDowell NG, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
McDowell NG, Allen CD, Marshall L (2010) Growth, carbon-isotope discrimination, and drought-associated mortality across a Pinus ponderosa elevational transect. Glob Chang Biol 16:399–415
McDowell NG, Bond BJ, Dickman LT, Ryan MG, Whitehead D (2011) Relationships between tree height and carbon isotope discrimination. In: Meinzer FC, Lachenbruch B, Dawson TE (eds) Size- and age-related changes in tree structure and function, pp 255–286
Michelot A, Eglin T, Dufrene E, Lelarge-Trouverie C, Damesin C (2011) Comparison of seasonal variations in water-use efficiency calculated from the carbon isotope composition of tree rings and flux data in a temperate forest. Plant Cell Environ 34:230–244
Penuelas J, Canadell JG, Ogaya R (2011) Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Glob Ecol Biogeogr 20:597–608
Rigling A, Bigler C, Eilmann B, Feldmeyer-Christe E, Gimmi U, Ginzler C, Graf U, Mayer P, Vacchiano G, Weber P, Wohlgemuth T, Zweifel R, Dobbertin M (2013) Driving factors of a vegetation shift from Scots pine to pubescent oak in dry Alpine forests. Glob Chang Biol 19:229–240
Sala A, Piper F, Hoch G (2010) Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol 186:274–281
Sarris D, Siegwolf R, Körner C (2013) Inter- and intra-annual stable carbon and oxygen isotope signals in response to drought in Mediterranean pines. Agric For Meteorol 168:59–68
Saurer M, Siegwolf RTW, Schweingruber FH (2004) Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years. Glob Chang Biol 10:2109–2120
Saurer M, Spahni R, Frank DC, Joos F, Leuenberger M, Loader NJ, McCarroll D, Gagen M, Poulter B, Siegwolf RTW, Andreu-Hayles L, Boettger T, Linan ID, Fairchild IJ, Friedrich M, Gutierrez E, Haupt M, Hilasvuori E, Heinrich I, Helle G, Grudd H, Jalkanen R, Levanic T, Linderholm HW, Robertson I, Sonninen E, Treydte K, Waterhouse JS, Woodley EJ, Wynn PM, Young GHF (2014) Spatial variability and temporal trends in water-use efficiency of European forests. Glob Chang Biol 20:3700–3712
Seibt U, Rajabi A, Griffiths H, Berry JA (2008) Carbon isotopes and water use efficiency: sense and sensitivity. Oecologia 155:441–454
Sitch S, Smith B, Prentice IC, Arneth A, Bondeau A, Cramer W, Kaplan JO, Levis S, Lucht W, Sykes MT, Thonicke K, Venevsky S (2003) Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Glob Chang Biol 9:161–185
Sun SJ, Qiu LF, He CX, Li CY, Zhang JS, Meng P (2018) Drought-affected Populus simonii Carr. show lower growth and long-term increases in intrinsic water-use efficiency prior to tree mortality. Forests 9
Szejner P, Belmecheri S, Ehleringer JR, Monson RK (2019) Recent increases in drought frequency cause observed multi-year drought legacies in the tree rings of semi-arid forests. Oecologia. https://doi.org/10.1007/s00442-00019-04550-00446
Thomas RB, Spal SE, Smith KR, Nippert JB (2013) Evidence of recovery of Juniperus virginiana trees from sulfur pollution after the clean air act. Proc Natl Acad Sci USA 110:15319–15324
Timofeeva G, Treydte K, Bugmann H, Rigling A, Schaub M, Siegwolf R, Saurer M (2017) Long-term effects of drought on tree-ring growth and carbon isotope variability in Scots pine in a dry environment. Tree Physiol 37:1028–1041
Treydte K, Frank D, Esper J, Andreu L, Bednarz Z, Berninger F, Böttger T, D‘Allessandro CD, Etien N, Filot M, Grabner M, Guillemin MT, Gutierrez E, Haupt M, Helle G, Hilasvuori E, Jungner H, Kalela-Brundin M, Krapiec M, Leuenberger M, Loader NJ, Masson-Delmotte V, Pazdur A, Pawelczyk S, Pierre M, Planells O, Pukiene R, Reynolds-Henne CE, Rinne KT, Saracino A, Saurer M, Sonninen E, Stievenard M, Switsur VR, Szczepanek M, Szychowska-Krapiec E, Todaro L, Waterhouse JS, Weigl M, Schleser GH (2007) Signal strength and climate calibration of a European tree ring isotope network. Geophys Res Lett 34. https://doi.org/10.1029/2007GL031106
Urrutia-Jalabert R, Malhi Y, Barichivich J, Lara A, Delgado-Huertas A, Rodriguez CG, Cuq E (2015) Increased water use efficiency but contrasting tree growth patterns in Fitzroya cupressoides forests of southern Chile during recent decades. J Geophys Res Biogeosciences 120:2505–2524
Vadeboncoeur MA, Jennings KA, Ouimette AP, Asbjornsen H (2020) Correcting tree-ring δ13C time series for tree-size effects in eight temperate tree species. Tree Physiol. https://doi.org/10.1093/treephys/tpz1138
Voelker SL, Muzika RM, Guyette RP (2008) Individual tree and stand level influences on the growth, vigor, and decline of red oaks in the Ozarks. For Sci 54:8–20
Voelker SL, Meinzer FC, Lachenbruch B, Brooks JR, Guyette RP (2014) Drivers of radial growth and carbon isotope discrimination of bur oak (Quercus macrocarpa Michx.) across continental gradients in precipitation, vapour pressure deficit and irradiance. Plant Cell Environ 37:766–779
Voelker SL, Roden JS, Dawson TE (2018) Millennial-scale tree-ring isotope chronologies from coast redwoods provide insights on controls over California hydroclimate variability. Oecologia 187:897–909
Voelker SL, Merschel AG, Meinzer FC, Ulrich DEM, Spies TA, Still CJ (2019) Fire deficits have increased drought sensitivity in dry conifer forests: Fire frequency and tree-ring carbon isotope evidence from Central Oregon. Glob Chang Biol 25:1247–1262
Voelker SL, Brooks JR, Meinzer FC, Anderson R, Bader MKF, Battipaglia G, Becklin KM, Beerling D, Bert D, Betancourt JL, Dawson TE, Domec JC, Guyette RP, Korner C, Leavitt SW, Linder S, Marshall JD, Mildner M, Ogee J, Panyushkina I, Plumpton HJ, Pregitzer KS, Saurer M, Smith AR, Siegwolf RTW, Stambaugh MC, Talhelm AF, Tardif JC, Van de Water PK, Ward JK, Wingate L (2016) A dynamic leaf gas-exchange strategy is conserved in woody plants under changing ambient CO2: evidence from carbon isotope discrimination in paleo and CO2 enrichment studies. Glob Chang Biol 22:889–902
Voelker SL, Wang SYS, Dawson TE, Roden JS, Still CJ, Longstaffe FJ, Ayalon A (2019b) Tree-ring isotopes adjacent to Lake Superior reveal cold winter anomalies for the Great Lakes region of North America. Sci Rep 9
Voltas J, Camarero JJ, Carulla D, Aguilera M, Ortiz A, Ferrio JP (2013) A retrospective, dual-isotope approach reveals individual predispositions to winter-drought induced tree dieback in the southernmost distribution limit of Scots pine. Plant Cell Environ 36:1435–1448
Wang SYS, Yoon JH, Becker E, Gillies R (2017) California from drought to deluge. Nat Clim Chang 7:465–468
Waterhouse JS, Switsur VR, Barker AC, Carter AHC, Hemming DL, Loader NJ, Robertson I (2004) Northern European trees show a progressively diminishing response to increasing atmospheric carbon dioxide concentrations. Quat Sci Rev 23:803–810
Yoon JH, Wang SYS, Gillies RR, Kravitz B, Hipps L, Rasch PJ (2015) Increasing water cycle extremes in California and in relation to ENSO cycle under global warming. Nat Commun 6
Young GHF, McCarroll D, Loader NJ, Gagen MH, Kirchhefer AJ, Demmler JC (2012) Changes in atmospheric circulation and the Arctic Oscillation preserved within a millennial length reconstruction of summer cloud cover from northern Fennoscandia. Clim Dyn 39:495–507
Acknowledgements
Steve Voelker was supported by US-NSF Award #1903721.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 © The Author(s)
About this chapter
Cite this chapter
Saurer, M., Voelker, S. (2022). Intrinsic Water-Use Efficiency Derived from Stable Carbon Isotopes of Tree-Rings. In: Siegwolf, R.T.W., Brooks, J.R., Roden, J., Saurer, M. (eds) Stable Isotopes in Tree Rings. Tree Physiology, vol 8. Springer, Cham. https://doi.org/10.1007/978-3-030-92698-4_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-92698-4_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92697-7
Online ISBN: 978-3-030-92698-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)