Abstract
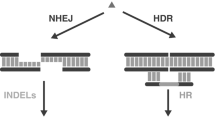
Targeting nucleases like zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and the clustered regularly interspaced short palindromic repeats/CRISPR-associated (CRISPR/Cas) system have revolutionized genome-editing possibilities in many model organisms. They allow the generation of loss-of-function alleles by the introduction of double-strand breaks at defined sites within genes, but also more sophisticated genome-editing approaches have become possible. These include the integration of donor plasmid DNA into the genome by homology-independent repair mechanisms after CRISPR/Cas9-mediated cleavage. Here we present a protocol outlining the most important steps to target a genomic site and to integrate a donor plasmid at this defined locus.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Walker C, Streisinger G (1983) Induction of mutations by gamma-rays in pregonial germ cells of zebrafish embryos. Genetics 103:125–136
Chakrabarti S, Streisinger G, Singer F, Walker C (1983) Frequency of gamma-ray induced specific locus and recessive lethal mutations in mature germ cells of the zebrafish, BRACHYDANIO RERIO. Genetics 103:109–123
Lin S, Gaiano N, Culp P, Burns JC, Friedmann T, Yee JK, Hopkins N (1994) Integration and germ-line transmission of a pseudotyped retroviral vector in zebrafish. Science 265:666–669
Mullins MC, Hammerschmidt M, Haffter P, Nusslein-Volhard C (1994) Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr Biol 4:189–202
Solnica-Krezel L, Schier AF, Driever W (1994) Efficient recovery of ENU-induced mutations from the zebrafish germline. Genetics 136:1401–1420
Amsterdam A, Hopkins N (1999) Retrovirus-mediated insertional mutagenesis in zebrafish. Methods Cell Biol 60:87–98
Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Malicki J, Stemple DL, Stainier DY, Zwartkruis F, Abdelilah S, Rangini Z et al (1996) A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123:37–46
Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, van Eeden FJ, Jiang YJ, Heisenberg CP et al (1996) The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123:1–36
Kawakami K, Shima A, Kawakami N (2000) Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proc Natl Acad Sci U S A 97:11403–11408
Kawakami K (2004) Transgenesis and gene trap methods in zebrafish by using the Tol2 transposable element. Methods Cell Biol 77:201–222
Kawakami K, Takeda H, Kawakami N, Kobayashi M, Matsuda N, Mishina M (2004) A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev Cell 7:133–144
Balciunas D, Ekker SC (2005) Trapping fish genes with transposons. Zebrafish 1:335–341
Balciuniene J, Balciunas D (2013) Gene trapping using gal4 in zebrafish. J Vis Exp (79):e50113
Balciuniene J, Nagelberg D, Walsh KT, Camerota D, Georlette D, Biemar F, Bellipanni G, Balciunas D (2013) Efficient disruption of Zebrafish genes using a Gal4-containing gene trap. BMC Genomics 14:619
Maddison LA, Li M, Chen W (2014) Conditional gene-trap mutagenesis in zebrafish. Methods Mol Biol 1101:393–411
Maddison LA, Lu J, Chen W (2011) Generating conditional mutations in zebrafish using gene-trap mutagenesis. Methods Cell Biol 104:1–22
Ni TT, Lu J, Zhu M, Maddison LA, Boyd KL, Huskey L, Ju B, Hesselson D, Zhong TP, Page-McCaw PS et al (2012) Conditional control of gene function by an invertible gene trap in zebrafish. Proc Natl Acad Sci U S A 109:15389–15394
Song G, Li Q, Long Y, Gu Q, Hackett PB, Cui Z (2012) Effective gene trapping mediated by Sleeping Beauty transposon. PLoS One 7:e44123
Song G, Li Q, Long Y, Hackett PB, Cui Z (2012) Effective expression-independent gene trapping and mutagenesis mediated by Sleeping Beauty transposon. J Genet Genomics 39:503–520
Scott EK, Mason L, Arrenberg AB, Ziv L, Gosse NJ, Xiao T, Chi NC, Asakawa K, Kawakami K, Baier H (2007) Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat Methods 4:323–326
le Trinh A, Fraser SE (2013) Enhancer and gene traps for molecular imaging and genetic analysis in zebrafish. Develop Growth Differ 55:434–445
Balciunas D, Davidson AE, Sivasubbu S, Hermanson SB, Welle Z, Ekker SC (2004) Enhancer trapping in zebrafish using the Sleeping Beauty transposon. BMC Genomics 5:62
Parinov S, Kondrichin I, Korzh V, Emelyanov A (2004) Tol2 transposon-mediated enhancer trap to identify developmentally regulated zebrafish genes in vivo. Dev Dyn 231:449–459
Choo BG, Kondrichin I, Parinov S, Emelyanov A, Go W, Toh WC, Korzh V (2006) Zebrafish transgenic Enhancer TRAP line database (ZETRAP). BMC Dev Biol 6:5
Kondrychyn I, Teh C, Garcia-Lecea M, Guan Y, Kang A, Korzh V (2011) Zebrafish enhancer TRAP transgenic line database ZETRAP 2.0. Zebrafish 8:181–182
Suster ML, Abe G, Schouw A, Kawakami K (2011) Transposon-mediated BAC transgenesis in zebrafish. Nat Protoc 6:1998–2021
Bussmann J, Schulte-Merker S (2011) Rapid BAC selection for tol2-mediated transgenesis in zebrafish. Development 138:4327–4332
Suster ML, Sumiyama K, Kawakami K (2009) Transposon-mediated BAC transgenesis in zebrafish and mice. BMC Genomics 10:477
Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA (2008) Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol 26:695–701
Doyon Y, McCammon JM, Miller JC, Faraji F, Ngo C, Katibah GE, Amora R, Hocking TD, Zhang L, Rebar EJ et al (2008) Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol 26:702–708
Huang P, Xiao A, Zhou M, Zhu Z, Lin S, Zhang B (2011) Heritable gene targeting in zebrafish using customized TALENs. Nat Biotechnol 29:699–700
Sander JD, Cade L, Khayter C, Reyon D, Peterson RT, Joung JK, Yeh JR (2011) Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat Biotechnol 29:697–698
Cade L, Reyon D, Hwang WY, Tsai SQ, Patel S, Khayter C, Joung JK, Sander JD, Peterson RT, Yeh JR (2012) Highly efficient generation of heritable zebrafish gene mutations using homo- and heterodimeric TALENs. Nucleic Acids Res 40:8001–8010
Dahlem TJ, Hoshijima K, Jurynec MJ, Gunther D, Starker CG, Locke AS, Weis AM, Voytas DF, Grunwald DJ (2012) Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet 8:e1002861
Moore FE, Reyon D, Sander JD, Martinez SA, Blackburn JS, Khayter C, Ramirez CL, Joung JK, Langenau DM (2012) Improved somatic mutagenesis in zebrafish using transcription activator-like effector nucleases (TALENs). PLoS One 7:e37877
Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh J-RJ, Joung JK (2013) Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol 31:227–229
Jao LE, Wente SR, Chen W (2013) Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc Natl Acad Sci U S A 110:13904–13909
Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD (2013) High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol 31:822–826
Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O et al (2013) DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 31:827–832
Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, Yang L, Church GM (2013) CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol 31:833–838
Pattanayak V, Lin S, Guilinger JP, Ma E, Doudna JA, Liu DR (2013) High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat Biotechnol 31:839–843
Sung YH, Kim JM, Kim HT, Lee J, Jeon J, Jin Y, Choi JH, Ban YH, Ha SJ, Kim CH et al (2014) Highly efficient gene knockout in mice and zebrafish with RNA-guided endonucleases. Genome Res 24(1):125–131
Gagnon JA, Valen E, Thyme SB, Huang P, Ahkmetova L, Pauli A, Montague TG, Zimmerman S, Richter C, Schier AF (2014) Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PLoS One 9:e98186
Liu D, Wang Z, Xiao A, Zhang Y, Li W, Zu Y, Yao S, Lin S, Zhang B (2014) Efficient gene targeting in zebrafish mediated by a zebrafish-codon-optimized cas9 and evaluation of off-targeting effect. J Genet Genomics 41:43–46
Auer TO, Del Bene F (2014) CRISPR/Cas9 and TALEN-mediated knock-in approaches in zebrafish. Methods 69(2):142–150
Shin J, Chen J, Solnica-Krezel L (2014) Efficient homologous recombination-mediated genome engineering in zebrafish using TALE nucleases. Development 141:3807–3818
Zu Y, Tong X, Wang Z, Liu D, Pan R, Li Z, Hu Y, Luo Z, Huang P, Wu Q et al (2013) TALEN-mediated precise genome modification by homologous recombination in zebrafish. Nat Methods 10:329–331
Irion U, Krauss J, Nusslein-Volhard C (2014) Precise and efficient genome editing in zebrafish using the CRISPR/Cas9 system. Development 141:4827–4830
Auer TO, Duroure K, De Cian A, Concordet JP, Del Bene F (2014) Highly efficient CRISPR/Cas9-mediated knock-in in zebrafish by homology-independent DNA repair. Genome Res 24:142–153
Kimura Y, Hisano Y, Kawahara A, Higashijima S (2014) Efficient generation of knock-in transgenic zebrafish carrying reporter/driver genes by CRISPR/Cas9-mediated genome engineering. Sci Rep 4:6545
Auer TO, Duroure K, Concordet JP, Del Bene F (2014) CRISPR/Cas9-mediated conversion of eGFP- into Gal4-transgenic lines in zebrafish. Nat Protoc 9:2823–2840
Rembold M, Lahiri K, Foulkes NS, Wittbrodt J (2006) Transgenesis in fish: efficient selection of transgenic fish by co-injection with a fluorescent reporter construct. Nat Protoc 1:1133–1139
Westerfield M (ed) (2000) The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio), 4th edn. University of Orgeon Press, Oregon
Foley JE, Maeder ML, Pearlberg J, Joung JK, Peterson RT, Yeh JR (2009) Targeted mutagenesis in zebrafish using customized zinc-finger nucleases. Nat Protoc 4:1855–1867
Hwang WY, Fu Y, Reyon D, Maeder ML, Kaini P, Sander JD, Joung JK, Peterson RT, Yeh JR (2013) Heritable and precise zebrafish genome editing using a CRISPR-Cas system. PLoS One 8:e68708
Montague TG, Cruz JM, Gagnon JA, Church GM, Valen E (2014) CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res 42:W401–W407
Naito Y, Hino K, Bono H, Ui-Tei K (2014) CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 31(7):1120–1123
Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DA (2004) Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol 22:589–594
Sambrook J, Russell DW (2006) Purification of nucleic acids by extraction with phenol:chloroform. CSH Protoc 2006
Di Donato V, Auer TO, Duroure K, Del Bene F (2013) Characterization of the calcium binding protein family in zebrafish. PLoS One 8:e53299
Abe G, Suster ML, Kawakami K (2011) Tol2-mediated transgenesis, gene trapping, enhancer trapping, and the Gal4-UAS system. Methods Cell Biol 104:23–49
Acknowledgments
A special thanks to J. P. Concordet, K. Duroure, and A. De Cian for helping with the development and initial establishment of the homology-independent targeting strategy. We would like to thank J. Wittbrodt for scientific discussion and support and members of the Del Bene lab for general discussion and comments. The Del Bene lab “Neural Circuits Development” is part of the Laboratoire d’Excellence (LabEx) entitled DEEP (ANR-11-LABX-0044) and the Ecole des Neurosciences de Paris. T.O.A. was supported by a Boehringer Ingelheim Fonds Ph.D. fellowship. This work has been supported by ATIP/AVENIR program starting grant (FDB), ERC-StG #311159 (FDB), CNRS, INSERM, and Institut Curie.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Auer, T.O., Del Bene, F. (2016). Homology-Independent Integration of Plasmid DNA into the Zebrafish Genome. In: Kawakami, K., Patton, E., Orger, M. (eds) Zebrafish. Methods in Molecular Biology, vol 1451. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3771-4_3
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3771-4_3
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3769-1
Online ISBN: 978-1-4939-3771-4
eBook Packages: Springer Protocols