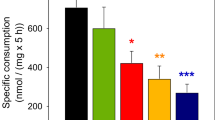
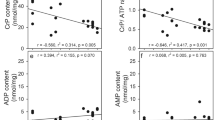
Abstract
Uptake of taurine by different tissues or cell types has been extensively studied these last decades (8,10,20). The high affinity taurine uptake displayed by the majority of the cells studied is a Na+, Cl− dependent system. Various hormones, drugs, and amino acids have been shown to affect this uptake. These compounds have been very often tested in vitro on cells in culture. However, they were usually tested by addition to the incubation media used for testing the taurine uptake. Some of these incubation media contain HEPES (N,N’hydroxyethylpiperazineethane sulfonate), which shows structural similarities with taurine (2-aminoethane sulfonate). We have previously shown that HEPES inhibits the taurine uptake of rat glial cells in primary cultures. This occurs by two mechanisms (11,12). A fast inhibition (within a few minutes) is observed when a Krebs-Ringer buffer supplemented with HEPES is used for the incubation of cells during taurine uptake determinations. On the contrary, when the glial cells are grown for a few days in a culture medium buffered with HEPES, instead of the more normal Na+ bicarbonate system, an inhibition of the taurine uptake is observed when cells are tested in the Krebs-Ringer incubation medium (11). This inhibition increases slowly with the culture length in the presence of HEPES (11). These results suggest the existence of two different mechanisms; a fast one (F) and a slow one (S), which may depend on the exposure time and conditions, and by which taurine uptake could be modulated. Different taurine analogs such as guanidinoethane sulfonate β-alanine, or hypotaurine are generally used to characterize the uptake of taurine by cultured cells by addition to the incubation media used for taurine uptake measurements. We have compared the effects obtained for these compounds on the taurine uptake upon addition to either the culture media (S condition) or the incubation media (F condition).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Benda, P., Lightbody, J., Sato, G., Levine, L., and Sweet, W. 1968, Differentiated rat glial cell strain in tissue culture, Science, 161:370–371.
Booher, J. and Sensenbrenner, M. 1972, Growth and cultivation of dissociated neurons and glial cells from embryonic chick, rat and human brain in flask cultures, Neurobiology, 2:97–105.
Borg, J., Balcar, V.J., Mark, J., and Mandel, P. 1979, Characterization of taurine uptake by neuronal and glial cells in culture, J.Neurochem., 32:1801–1805.
Field, L., Owen, T.C., Crenshaw, R.R., and Bryan, A.W. 1961, Organic disulfids and related substances. Thiosulfonates and disulfides containing 2-aminoethyl moieties, J.Amer.Chem.Soc., 83:4414–4417.
Gray, R.G., Pollit, R.J., and Webley, J. 1987, Methylmalonic semialdehyde dehydrogenase deficiency: demonstration of defective valine and β-alanine metabolism and reduced malonic semialdehyde dehydrogenase activity in cultured fibroblasts, Biochem.Med.Metab.Biol., 38:121–124.
Holopainen, I., Malminen, O., and Kontro, P. 1988, Sodium-dependent high-affinity uptake of taurine in cultured cerebellar granule cells and astrocytes, J Neurosci Res, 18:479–483.
Holopainen, L. and Kontro, P. 1986, High-affinity uptake of taurine and beta-alanine in primary cultures of rat astrocytes, Neurochem.Res., 11:207–215.
Huxtable, R.J. 1989, Taurine in the central nervous system and the mammalian actions of taurine, Prog.Neurobiol., 32:471–533.
Huxtable, R.J., Laird, H.E., and Lippincott, S.E. 1979, The transport of taurine in the heart and the rapid depletion of tissue taurine content by guanidinoethyl sulfonate, Journal of Pharmacology and Experimental Therapeutics, 211:465–471.
Jacobsen, J.G. and Smith, L.H. 1968, Biochemistry and physiology of taurine and taurine derivatives, Physiol.Rev., 48:424–511.
Lleu, P.L. and Rebel, G. 1989, Effect of HEPES on the taurine uptake by cultured glial cells, J.Neurosci.Res., 23:78–86.
Lleu, P.L. and Rebel, G. 1990, Effect of HEPES on the Na+,Cl−-dependent uptake of taurine and α-alanine by cultured glial cells. Modulation by composition and osmolarity of medium, Neuropharmacology, 29:719–725.
Lowry, O.J., Rosebrough, N.J., Farr, A.L., and Randall, R.J. 1951, Protein measurement with folin phenol reagent, J.Biol.Chem., 193:265–275.
Martin, D.L. and Shain, W. 1979, High affinity transport of taurine and β-alanine and low affinity transport of r-aminobutyric acid by a single transport system in cultured glioma cells, J.Biol. Chem., 254:7076–7084.
Mitchell, D.B., Santone, K.S., and Acosta, D. 1980, Evaluation of cytotoxicity in cultured cells by enzyme leakage, J.Tissue Cult.Meth., 6:113–116.
Nutzenadel, W. and Scriver, C.R. 1976, Uptake and metabolism of β-alanine and L-carnosine by rat tissues in vitro: role of nutrition, Am.J.Physiol., 230:643–651.
Schousboe, A., Fosmark, H., and Svenneby, G. 1976, Taurine uptake in astrocytes cultured from dissociated mouse brain hemispheres, Brain Res., 116:158–164.
Schrier, B.K. and Thompson, E.J. 1974, Uptake, excretion and metabolism of putative neurotransmitters by cultured glial tumor cells, J. Biol. Chem., 249:1769–1780.
Tasaka, J., Sakai, S., Tosaka, T., and Yoshihama, I. 1989, Glial uptake system of GABA distinct from that of taurine in the bullfrog sympathetic ganglia, Neurochem.Res., 14:271–277.
Wright, C.E., Tallan, H.H., Lin, Y.Y., and Gaull, G.E. 1986, Taurine: biological update, Ann.Rev.Biochem., 55:427–453.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1994 Springer Science+Business Media New York
About this chapter
Cite this chapter
Rebel, G., Petegnief, V., Lleu, PL., Gupta, R.C., Guérin, P., Bourguignon, J. (1994). New Data on the Regulation of Taurine Uptake in Cultured Nervous Cells. In: Huxtable, R.J., Michalk, D. (eds) Taurine in Health and Disease. Advances in Experimental Medicine and Biology, vol 359. Springer, Boston, MA. https://doi.org/10.1007/978-1-4899-1471-2_23
Download citation
DOI: https://doi.org/10.1007/978-1-4899-1471-2_23
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4899-1473-6
Online ISBN: 978-1-4899-1471-2
eBook Packages: Springer Book Archive