Abstract
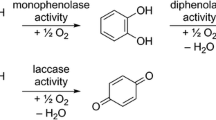
Attempts to characterize tannin activity in biological systems have met with mixed success. Although there are many examples in which tannins inhibit fungal, bacterial, and/or viral growth, there are also many failures to find such activity. It has proven especially difficult to characterize tannin activity in insect herbivores, even when polyphenols comprise a large fraction of the diet and the insect appears to suffer as a consequence. The affinity of polyphenols for various substrates (e.g., proteins) varies with the physiochemical conditions in which the complexation or reaction takes place. We postulate that variation among insect species in midgut conditions, mainly redox potential and pH, yields variable impacts of phenolics on insects. Redox conditions and pH are influenced by intrinsic physiological characteristics of the insect and by foliar oxidative enzymes, non-enzymatic oxidants, and reductants. Many insects appear to possess adaptations that relate directly to the oxidative state of ingested phenolics. In many cases, oxidative activation may be necessary before biological impacts can be observed.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Harborne, J.B. Flavonoid pigments. In: Rosenthal, G.A.; Janzen, D.H. (eds.) Herbivores: their interactions with secondary plant metabolites. Academic Press, New York, p. 619 (1979).
Harborne, J.B. Nature, distribution, and function of plant flavonoids. In: Cody, V.; Middleton, E. Jr.; J. Harborne, J.B. (eds.) Plant flavonoids in biology and medicine. Alan R. Liss, New York, p. 15 (1986).
Swain, T. Tannins and lignins. In: Rosenthal, G.A.; Janzen, D.H. (eds.) Herbivores: their interactions with secondary plant metabolites. Academic Press, New York, p. 657 (1979).
Feeny, P.P. Plant apparency and chemical defense. Recent Adv. Phytochemistry 10:1 (1976).
Rhoades, D.F.; Cates, R.G. Towards a general theory of plant antiherbivore chemistry. Recent Adv. Phytochemistry 10:168 (1976).
Coley, P.D.; Bryant, J.P.; Chapin, F.S. III. Resource availability and plant antiherbivore defense. Science 230:895 (1985).
Schultz, J.C. Tannin-insect interactions. In: Hemingway, R.W.; Karchesy, J. J. (eds.) Chemistry and significance of condensed tannins. Plenum Press, New York, p. 417 (1989).
Felton, G.W.; Duffey, S.S. Inactivation of baculovirus by quinones formed in insect-damaged plant tissues. J. Chem. Ecol. 16:1221 (1990).
Hedin, P.A.; Lindig, O.H.; Sikorowski, P.P.; Wyatt, M. Suppressants of gut bacteria in the boll weevil from the cotton plant. J. Econ. Entomol. 71:294 (1978).
Koike, S.; Iizuka, T.; Mizutani, J. Determination of caffeic acid in the digestive juice of silkworm larvae and its antibacterial activity against the pathogenic Streptoccus faecalis AD-4. Agric. Biol. Chem. Jpn. 43:1727 (1979).
Ludlum, CT.; Feiton, G.W.; Duffey, S.S. Plant defenses: chlorogenic acid and polyphenol oxidase enhance toxicity of Bacillus thuringiensis suhsp. kurstaki to Heliothis zea. J. Chem. Ecol. 17:217 (1991).
Schultz, J.C; Keating, S.T. Host-plant mediated interactions between the gypsy moth and a baculovirus. In: Barbosa, P.; Krischik, V.A.; Jones, C.G. (eds.) Microbial mediation of plant-herbivore interactions. Wiley and Sons, New York, p. 489 (1991).
Taper, M.L; Zimmerman, E.R.; Case, T.J. Sources of mortality for a cynipid gall-wasp (Dryocosmus dubiosus (Hymenoptera: Cynipidae)): the importance of the tannin/fungus interaction. Oecologia 68:437 (1986).
Feeny, P.P. Effect of oak leaf tannins on larval growth of the winter moth Operophtera brumata. J. Insect Physiol. 14:805 (1968).
Feeny, P. P. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51:561 (1970).
Bernays, E.A. Tannins: an alternative viewpoint. Ent. Exp. Appl. 24:44 (1978).
Bernays, E.A. Plant tannins and insect herbivores: an appraisal. Ecol. Entomol. 6:353 (1981).
Martin, M.M.; Martin, J.S. Surfactants: their role in preventing the precipitation of proteins in insect guts. Oecologia 61:342 (1984).
Martin, J.S.; Martin, M.M.; Bernays, E.A. Failure of tannic acid to inhibit digestion or reduce digestibility of plant protein in gut fluids of insect herbivores: implication for theories of plant defense. J. Chem. Ecol. 13:605 (1987).
Loomis, W.D. Overcoming problems of phenolic and quinones in the isolation of plant enzymes and organelles. Methods Enzym. 54:528 (1974).
Hagerman, A.E.; Klucher, K.M. Tannin-protein interactions. In: Cody, V.; Middleton, E. Jr.; Harborne, J.B. (eds.) Plant flavonoids in biology and medicine. Alan R. Liss, New York, p. 67 (1986).
Hagerman, A.E. Chemistry of tannin-protein complexation. In: Hemingway, R.W.; Karchesy, J.J. (eds.) Chemistry and significance of condensed tannins. Plenum Press, New York, p. 323 (1989).
Ya, C; Gaffney, S.H.; Lilley, T.H.; Haslam, E. Carbohydrate-polyphenol complexation. In: Hemingway, R.W.; Karchesy, J.J. (eds.) Chemistry and significance of condensed tannins. Plenum Press, New York, p. 307 (1989).
Appel, H.M.; Martin, M.M. Redox conditions in herbivorous lepidopteran larvae. J. Chem. Ecol. 12:3277 (1990).
Duffey, S.S.; Felton, G.W. Plant enzymes in resistance to insects. In: Whitaker, J.R.; Sonnett, P.E. (eds.) Biocatalysis in agricultural biotechnnology. Amer. Chemical Society, Symposium Series, Washington, D.C., p. 289 (1989).
Hagerman, A.E.; Robbins, C.T. Implication of soluble tannin-protein complexes for tannin analysis and plant defense mechanisms. J. Chem. Ecol. 13:1243 (1987).
Oh, H.I.; Hoff, J.E.; Armstrong, G.S.; Haff, L.A. Hydrophobic interaction in tannin-protein complexes. J. Agric. Food Chem. 28:394 (1980).
Martin, M.M.; Rockhohn, D.D.; Martin, J.S. Effects of surfactants, pH, and certain cations on precipitation of proteins by tannins. J. Chem. Ecol. 11:485 (1987).
Schultz, J.C.; DeVeau, E. Reassessment of the interaction between gut detergents and phenolics in Lepidoptera and significance for gypsy moth larvae, [submitted to J. Chem. Ecol.].
Berenbaum, M. Adaptive significance of midgut pH in larval Lepidoptera. Am.Nat. 115:138 (1980).
Martin, M.M.; Kukor, J.J.; Martin, J. S.; Lawson, D.L.; Merritt, R.W. Digestive enzymes of larvae of three species of caddisflies (Trichoptera). Insect Biochem. 11:501 (1981).
Martin, M.M.; Martin, J.S.; Kukor, J.J.; Merritt, R.W. The digestive enzymes of detritus-feeding stonefly nymphs (Plecoptera: Pteronarcyidae). Can. J. Zool. 59:1947 (1981).
Schultz, J.C.; Lechowicz, M.J. Hostplant, larval age and feeding behavior influence midgut pH in the gypsy moth (Lymantria dispar). Oecologia 71:153 (1986).
Appel, H.M.; Schultz, J.C.; [unpublished results].
Hemingway, R.W. Reactions at the interflavanoid bond of proanthocyanidins. In: Hemingway, R.W.; Karchesy, J.J. (eds.) Chemistry and significance of condensed tannins. Plenum Press, New York, p. 265 (1989).
Keating, S.T.; Schultz, J.C.; Yendol, W.G. The effect of diet on gypsy moth (Lymantria dispar) larval midgut pH, and its relationship with larval susceptibility to a baculovirus. J. Invert Path. 56:317 (1990).
MacManus, J.P.; Davis, K.G.; Beart, J.E.; Gaffney, S.H.; Lilley, T.H.; Haslam, E. Polyphe-nol interactions. Part 1. Introduction; some observations on the reversible complexation of polyphenols with proteins and polysaccharides.J. Chem.Soc. Perkin Trans. 2:1429 (1985).
Felton, G.W.; Donato, K., Del Vecchio, R.J., Duffey, S.S. Activation of plant foliar oxidases by insect feeding reduces nutritive quality of foliage for noctuid herbivores. J. Chem. Ecol. 15:2667 (1989).
Cheynier, V.; Basire, N.; Rigaud, J. Mechanism of trans-caffeoyltartaric acid and catechin oxidation in model solutions containing grape polyphenoloxidase. J. Agric. Food Chem. 37:1069 (1989).
Cheynier, V.; Rigaud, J.; Moutounet, M. Oxidation kinetics of trans-caffeoyltartrate and its glutathione derivatives in grape musts. Phyto chemistry 29:1751 (1990).
Miles, P.W.; Peng, Z. Studies on the salivary physiology of plant bugs: detoxification of phytochemicals by the salivary peroxidase of aphids. J. Insect Physiol. 35:865 (1989).
Peng, Z.; Miles, P. Acceptability of catechin and its oxidative condensation products to the rose aphid, Macrosiphum rosac. Ent. Exp. & Appl. 47:255 (1988).
Peng, Z.; Miles, P. Studies on the salivary physiology of plant bugs: function of the catechol oxidase of the rose aphid. J. Insect Physiol. 34:1027 (1988).
Larson, R.A. The antioxidants of higher plants. Phyto chemistry 27:969 (1988).
Smith, M.T. Quinones as mutagens, carcinogens, and anticancer agents: introduction and overview. J. Toxic. Environ. Health 16:665 (1985).
Kappus, H. Overview of enzyme systems involved in bioreduction of drugs and in redox cycling. Biochem. Pharm. 35:1 (1986).
Ahmad, S.; Pritsos, CA.; Bowen, S.M.; Kirkland, K.E.; Blomquist, G.J.; Pardini, R.S. Activities of enzymes that detoxify Superoxide anion and related toxic oxyradicals in Trichoplusia ni. Arch. Biochem. Physiol. 6:85 (1987).
Ahmad, S.; Pristos, CA.; Bowen, S.M.; Heisler, C.R.; Blomquist, G.J.; Pardini, R.S. Antioxidant enzyzmes of larvae of the cabbage looper moth, Trichoplusia ni: subcellular distribution and activities of Superoxide dismutase, catalase, and glutathione reductase. Free Rad. Res.Comms. 4:403 (1988).
Ahmad, S.; Pardini, R.S. Antioxidant defense of the cabbage looper, Trichoplusia ni-enzymatic responses to the superoxide-generating flavonoid, quercetin, and photodynamic furanocoumarin, xanthotoxin. Photochem. Photobiol. 51:305 (1990).
Aucoin, R.R.; Philogene, B.J.R.; Arnason, J.T. Antioxidant enzymes as biochemical defenses against phototoxin-induced oxidative stress in three species of herbivorous Lepidoptera. Arch. Insect Biochem. Physiol. 16:139 (1991).
Lee, K.; Berenbaum, M.R. Action of antioxidant enzymes and cytochrome P-450 monooxy-genases in the cabbage looper in response to plant phototoxins. Arch. Insect Biochem. Physiol. 10:151 (1989).
Lee, K.; Berenbaum, M.R. Defense of parsnip webworm against phototoxic furanocourmarins: role of antioxidant enzymes. J. Chem. Ecol. 16:2451 (1990).
Pristos, CA.; Ahmad, S.; Bowen, S.M.; Blomquist, G.J.; Pardini, R.S. Antioxidant enzyme activities in the southern armyworm, Spodoptera eridania. Comp.Biochem.Physiol. 900:423 (1988).
Pristos, CA.; Pastore, J.; Pardini, R.S. Role of Superoxide dismutase in the protection and tolerance to the prooxidant allelochemical quercetin in Papilio polyxenes, Spodoptera eridania, and Trichoplusia ni. Arch. Biochem. Physiol. 16:273 (1991).
Felton, G.W.; Duffey, S.S. Ascorbate oxidation-reduction in Helicoverpa zea as a scavenging system against dietary oxidants. Arch. Insect Biochem. Pkysiol. 19:27–37 (1992).
Steinly, B.A.; Berenbaum, M. Histopathological effects of tannins on the midgut epithelium of Papilio polyxenes and Papilio glaucus. Ent. Exp. Appl. 39:3 (1985).
Barbosa, P.; Krischik, V.A. Influence of alkaloids on feeding preference for Eastern deciduous forest trees by the gypsy moth, Lymantria dispar. Am Nat. 130:87 (1988).
Bate-Smith, E.C. Astringent tannins of Acer species. Phytochemistry 16:1421 (1977).
Schultz, J.C.; Baldwin, I.T. Oak leaf quality declines in response to defoliation by gypsy moth larvae. Science 217:149 (1982).
Bate-Smith, E.C. Phytochemistry of proanthocyanidins. Phyto chemistry. 14:1107 (1975).
Rossiter, M.C.; Schultz, J.C.; Baldwin, I.T. Relationships among defoliation, red oak phenolics, and gypsy moth growth and reproduction. Ecology 69:267 (1988).
Kleiner, K.; Montgomery, M.E. Opposing relationships between gypsy moth performance and tannins of two oak species, [in press].
Keating, S.T.; Yendol, W.G.; Schultz, J.C. Relationship between susceptibility of gypsy moth larvae (Lepidoptera:Lymantriidae) to a baculovirus and host plant foliage constituents. Environ. Entomol. 17:952 (1988).
Keating, S.T.; McCarthy, W.J.; Yendol, W.G. Gypsy moth (Lymantria dispar) larval susceptibility to a baculovirus affected by selected nutrients, hydrogen ions (pH), and plant allelochemicals in artificial diets. J. Inver. Path. 54:165 (1989).
Schultz, J.C.; Foster, M.A.; Montgomery, M.E. Host plant-mediated impacts of a baculovirus on gypsy moth populations. In: Watt, A.D.; Leather S.R; Hunter, M.D.; Kidd, N.A.C. (eds.) Population dynamics of forest insects. Intercept, U.K. p. 303 (1990).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1992 Springer Science+Business Media New York
About this chapter
Cite this chapter
Appel, H.M., Schultz, J.C. (1992). Activity of Phenolics in Insects: The Role of Oxidation. In: Hemingway, R.W., Laks, P.E. (eds) Plant Polyphenols. Basic Life Sciences, vol 59. Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-3476-1_34
Download citation
DOI: https://doi.org/10.1007/978-1-4615-3476-1_34
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4613-6540-2
Online ISBN: 978-1-4615-3476-1
eBook Packages: Springer Book Archive