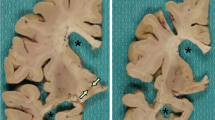
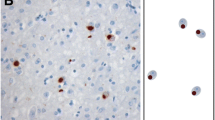
Abstract
Picks disease is a major clinicopathological disease having circumscribed atrophy in the frontotemporal lobe. Demented patients with frontotemporal atrophy are now clinically diagnosed as frontotemporal lobar degeneration (FTLD). Other underlying pathologies in patients with FTLD include FTLD with TDP-43-positive inclusions, corticobasal degeneration, progressive supranuclear palsy, basophilic inclusion body disease, neuronal intermediate filament inclusion disease and argyrophilic grain disease. In this chapter, recent findings regarding the distinct clinical and histopathological features of these pathological disease entities are presented including the discussion on the possibility of future antemortem diagnosis of patients with the disease.
In this chapter, recent findings regarding the distinct clinical and histopathological features of these pahtological disease entities are presented including the discussion on the possibility of future antemortem diagnosis of patients with the disease.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Pick A. Ueber die Beziehungen der senilen Hirnatrophie zur Aphasie. Prag Med Wochenschr 1892; 17: 165–167.
Alzheimer A. Über eigenartige Krankheitfälle des spätern Alters. Z Ges Neurol Psychiatr 1911; 4: 356–385.
Onari K, Spatz H. Anatomische Beiträge zur Lehre von pickschen umschriebenen Größhirnrinden Atrophie (Picksche Krankheit). Z Ges Neurol Psychiatr 1926; 101:470–511.
Cummings JL, Duchen LW. Klüver-Bucy syndrome in Pick disease: clinical and pathologic correlations. Neurology 1981; 31:1415–1422.
The Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry 1994; 57:416–418.
Neary D, Snowden JS, Gustafson L et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51:1546–1554.
Gibb WR, Luthert PJ, Marsden CD. Corticobasal degeneration. Brain 1989; 112:1171–1192.
Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol 1964; 10:333–359.
Braak H, Braak E. Argyrophilic grains: characteristic pathology of cerebral cortex in cases of adult onset dementia without Alzheimer changes. Neurosci Lett 1987; 76:124–127.
Munoz-Garcia D, Ludwin SK. Classic and generalized variants of Pick’s disease: a clinicopathological, ultrastructural and immunocytochemical comparative study. Ann Neurol 1984; 16:467–480.
Cairns NJ, Perry RH, Jaros E et al. Patients with a novel neurofilamentopathy: dementia with neurofilament inclusions. Neurosci Lett 2003; 341:177–180.
Josephs KA, Holton JL, Rossor MN et al. Neurofilament inclusion body disease: a new proteinopathy. Brain 2003; 126:2291–2303.
Arai T, Hasegawa M, Akiyama H et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 2006; 351:602–611.
Neumann M, Sampathu DM, Kwong LK et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006; 314:130–133.
Jackson M, Lennox G, Lowe J. Motor neuron disease-inclusion dementia. Neurodegeneration 1996; 5:339–350.
Cairns NJ, Bigio EH, Mackenzie IR et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathol 2007; 114:5–22.
Mackenzie IR, Neumann M, Bigio EH et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol 2009; 117:15–18.
Cairns NJ, Grossman M, Arnold SE et al. Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease. Neurology 2004; 63:1376–1384.
Munoz DG, Neumann M, Kusaka H et al. FUS pathology in basophilic inclusion body disease. Acta Neuropathol 2009; 118:617–627.
Neumann M, Roeber S, Kretzschmar HA et al. Abundant FUS-immunoreactive pathology in neuronal intermediate filament inclusion disease. Acta Neuropathol 2009; 118:605–616.
Knopman DS, Mastri AR, Frey WH 2nd et al. Dementia lacking distinctive histologic features: a common non-Alzheimer degenerative dementia. Neurology 1990; 40:251–256.
Mackenzie IR, Shi J, Shaw CL et al. Dementia lacking distinctive histology (DLDH) revisited. Acta Neuropathol 2006; 112:551–559.
Munoz DG, Dickson DW, Bergeron C et al. The neuropathology and biochemistry of frontotemporal dementia. Ann Neurol 2003; 54 Suppl 5:s24–s28.
Josephs KA, Holton JL, Rossor MN et al. Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathol Appl Neurobiol 2004; 30:369–373.
Forman MS, Farmer J, Johnson JK et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol 2006; 59:952–962.
Ikeda K. Neuropathological discrepancy between Japanese Pick’s disease without Pick bodies and frontal lobe degeneration type of frontotemporal dementia proposed by Lund and Manchester Group. Neuropathology 2000; 20:76–82.
Mackenzie IR, Neumann M, Bigio EH et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 2010; 119:1–4.
Mackenzie IR, Foti D, Woulfe J et al. Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain 2008; 131:1282–1293.
Neumann M, Rademakers R, Roeber S et al. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain 2009; 132:2922–2931.
Frank S, Tolnay M. Frontotemporal lobar degeneration: toward the end of confusion. Acta Neuropathol 2009; 118:629.
Azmador-Ortiz C, Lin WL, Ahmed Z et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 2007; 61:435–445.
Arai T, Mackenzie IR, Hasegawa M et al. Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol 2009; 117:125–136.
Higashi S, Iseki E, Yamamoto R et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res 2007; 1184:284–294.
Nakashima-Yasuda H, Uryu K et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol 2007; 114:221–229.
Yokota O, Davidson Y, Arai T et al. Effect of topographical distribution of alpha-synuclein pathology on TDP-43 accumulation in Lewy body disease. Acta Neuropathol 2010, in press.
Uryu K, Nakashima-Yasuda H, Forman MS et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 2008; 67:555–564.
Hasegawa M, Arai T, Akiyama H et al. TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain 2007; 130:1386–1394.
Geser F, Winton MJ, Kwong LK et al. Pathological TDP-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol 2008; 115(1):133–145.
Fujishiro H, Uchikado H, Arai T et al. Accumulation of phosphorylated TDP-43 in brains of patients with argyrophilic grain disease. Acta Neuropathol 2009; 117:151–158.
Yokota O, Davidson Y, Bigio EH et al. Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol 2010; 120:55–66.
Geser F, Martinez-Lage M, Kwong LK et al. Amyotrophic lateral sclerosis, frontotemporal dementia and beyond: the TDP-43 diseases. J Neurol 2009; 256(8):1205–1214.
Chen-Plotkin AS, Lee VM, Trojanowski JQ. TAR DNA-binding protein 43 in neurodegenerative disease. Nat Rev Neurol 2010; 6(4):211–220.
Josephs KA, Whitwell JL, Knopman DS et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology 2008; 70:1850–1857.
Ikeda M, Ishikawa T, Tanabe H. Epidemiology of frontotemporal lobar degeneration. Dement Geriatr Cogn Disord 2004; 17:265–268.
Yokota O, Sasaki K, Fujisawa Y et al. Frequency of early and late-onset dementias in a Japanese memory disorders clinic. Eur J Neurol 2005; 12:782–790.
Hodges JR, Davies RR, Xuereb JH et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol 2004; 56:399–406.
Lipton AM, White CL 3rd, Bigio EH. Frontotemporal lobar degeneration with motor neuron disease-type inclusions predominates in 76 cases of frontotemporal degeneration. Acta Neuropathol 2004; 108:379–385.
Kertesz A, McMonagle P, Blair M et al. The evolution and pathology of frontotemporal dementia. Brain 2005; 128:1996–2005.
Shi J, Shaw CL, Du Plessis D et al. Histopathological changes underlying frontotemporal lobar degeneration with clinicopathological correlation. Acta Neuropathol 2005; 110:501–512.
Yokota O, Tsuchiya K, Arai T et al. Clinicopathological characterization of Pick’s disease versus frontotemporal lobar degeneration with ubiquitin/TDP-43-positive inclusions. Acta Neuropathol 2009; 117: 429–444.
Snowden JS, Pickering-Brown SM, Mackenzie IR et al. Progranulin gene mutations associated with frontotemporal dementia and progressive nonfluent aphasia. Brain 2006; 129:3091–3102.
King ME, Ghoshal N, Wall JS et al. Structural analysis of Pick’s disease-derived and in vitro-assembled tau filaments. Am J Pathol 2001; 158(4):1481–1490.
Odawara T, Iseki E, Kanai A et al. Clinicopathological study of two subtypes of Pick’s disease in Japan. Dement Geriatr Cogn Disord 2003; 15:19–25.
Davies RR, Hodges JR, Kril JJ et al. The pathological basis of semantic dementia. Brain 2005; 128: 1984–1995.
Godbolt AK, Josephs KA, Revesz T et al. Sporadic and familial dementia with ubiquitin-positive tau-negative inclusions: clinical features of one histopathological abnormality underlying frontotemporal lobar degeneration. Arch Neurol 2005; 62:1097–1101.
Grimes DA, Bergeron CB, Lang AE. Motor neuron disease-inclusion dementia presenting as cortical-basal ganglionic degeneration. Mov Disord 1999; 14:674–780.
Paviour DC, Lees AJ, Josephs KA et al. Frontotemporal lobar degeneration with ubiquitin-onlyimmunoreactive neuronal changes: broadening the clinical picture to include progressive supranuclear palsy. Brain 2004; 127:2441–2451.
Graham A, Davies R, Xuereb J et al. Pathologically proven frontotemporal dementia presenting with severe amnesia. Brain 2005; 128:597–605.
Claassen DO, Parisi JE, Giannini C et al. Frontotemporal dementia mimicking dementia with Lewy bodies. Cogn Behav Neurol 2008; 21(3):157–163.
Josephs KA, Ahmed Z, Katsuse O et al. Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. J Neuropathol Exp Neurol 2007; 66:142–151.
Pickering-Brown SM, Rollinson S, Du Plessis D et al. Frequency and clinical characteristics of progranulin mutation carriers in the Manchester frontotemporal lobar degeneration cohort: comparison with patients with MAPT and no known mutations. Brain 2008; 131:721–731.
Le Ber I, Camuzat A, Hannequin D et al. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain 2008; 131:732–746.
Fukui T, Sugita K, Kawamura M et al. Primary progressive apraxia in Pick’s disease: a clinicopathologic study. Neurology 1996; 47:467–473.
Kawamura M, Mochizuki S. Primary progressive apraxia. Neuropathology 1999; 19:249–258.
Tsuchiya K, Ikeda K, Uchihara T et al. Distribution of cerebral cortical lesions in corticobasal degeneration: a clinicopathological study of five autopsy cases in Japan. Acta Neuropathol 1997; 94:416–424.
Grimes DA, Lang AE, Bergeron CB. Dementia as the most common presentation of cortical-basal ganglionic degeneration. Neurology 1999; 53:1969–1974.
Joseph KA, Petersen RC, Knopman DS et al. Clinicopathological analysis of frontotemporal and corticobasal degenerations and PSP. Neurology 2006; 66:41–48.
Josephs KA, Duffy JR. Apraxia of speech and nonfluent aphasia: a new clinical marker for corticobasal degeneration and progressive supranuclear palsy. Current Opinion in Neurology 2008; 21:688–692.
Ikeda K, Akiyama H, Iritani S et al. Corticobasal degeneration with primary progressive aphasia and accentuated cortical lesion in superior temporal gyrus: case report and review. Acta Neuropathol 1996; 92:534–539.
Tsuchiya K, Murayama S, Mitani K et al. Constant and severe involvement of Betz cells in corticobasal degeneration is not consistent with pyramidal signs: a clinicopathological study of ten autopsy cases. Acta Neuropathol 2005; 109:353–366.
Boeve BF, Maraganore DM, Parisi JE et al. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology 1999; 53:795–800.
Yokota O, Tsuchiya K, Terada S et al. Basophilic inclusion body disease and neuronal intermediate filament inclusion disease: a comparative clinicopathological study. Acta Neuropathol 2008; 115:561–575.
Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol 2009; 8:270–279.
Kusaka H, Matsumoto S, Imai T. An adult-onset case of sporadic motor neuron disease with basophilic inclusions. Acta Neuropathol 1990; 80:660–665.
Kusaka H, Matsumoto S, Imai T. Adult-onset motor neuron disease with basophilic intraneuronal inclusion bodies. Clin Neuropathol 1993; 12:215–218.
Ishihara K, Araki S, Ihori N et al. An autopsy case of frontotemporal dementia with severe dysarthria and motor neuron disease showing numerous basophilic inclusions. Neuropathology 2006; 26: 447–454.
Togo T, Sahara N, Yen SH et al. Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol 2002; 61(6):547–556.
Togo T, Cookson N, Dickson DW. Argyrophilic grain disease: neuropathology, frequency in a dementia brain bank and lack of relationship with apolipoprotein E. Brain Pathol 2002; 12:45–52.
Saito Y, Ruberu NN, Sawabe M et al. Staging of argyrophilic grains: an age-associated tauopathy. J Neuropathol Exp Neurol 2004; 63:911–918.
Tsuchiya K, Mitani K, Arai T et al. Argyrophilic grain disease mimicking temporal Pick’s disease: a clinical, radiological and pathological study of an autopsy case with a clinical course of 15 years. Acta Neuropathol 2001; 102:195–199.
Ishihara K, Araki S, Ihori N et al. Argyrophilic grain disease presenting with frontotemporal dementia: a neuropsychological and pathological study of an autopsied case with presenile onset. Neuropathology 2005; 25:165–170.
Ikeda K, Akiyama H, Arai T et al. Clinical aspects of argyrophilic grain disease. Clin Neuropathol 2000; 19:278–284.
Togo T, Isojima D, Akatsu H et al. Clinical features of argyrophilic grain disease: a retrospective survey of cases with neuropsychiatric symptoms. Am J Geriatr Psychiatry 2005; 13:1083–1091.
Uchikado H, Tsuchiya K, Tominaga I et al. Argyrophilic grain disease clinically mimicking Parkinson’s disease with dementia: report of an autopsy case. Brain and Nerve 2004; 56:785–788 (in Japanese with English abstract).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Landes Bioscience and Springer Science+Business Media
About this chapter
Cite this chapter
Takeda, N., Kishimoto, Y., Yokota, O. (2012). Pick’s Disease. In: Ahmad, S.I. (eds) Neurodegenerative Diseases. Advances in Experimental Medicine and Biology, vol 724. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-0653-2_23
Download citation
DOI: https://doi.org/10.1007/978-1-4614-0653-2_23
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-0652-5
Online ISBN: 978-1-4614-0653-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)