Abstract
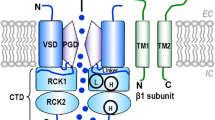
A role for integrins in mechanotransduction has been suggested because these molecules form an important mechanical link between the extracellular matrix (ECM) and the cytoskeleton. An example of mechanotransduction in blood vessels is the myogenic response—the rapid and maintained constriction of arterioles in response to pressure elevation. L-type calcium channels and large-conductance, calcium-activated potassium (BK) channels are known to play important roles in the myogenic response and in the maintenance of myogenic (pressure-induced) vascular tone. Our recent studies on isolated, cannulated arterioles and freshly-dispersed arteriolar smooth muscle cells show that both L-type calcium channels (Cav1.2) and BK channels are regulated by α5β1 integrin activation. α5β1 integrin interacts with the ECM protein fibronectin, which is distributed in basement membrane and interstitial matrices surrounding smooth muscle cells within the arteriolar wall. Truncation and site-directed mutagenesis strategies reveal that regulation of Cav1.2 by α5β1 integrin requires phosphorylation of the channel α1C subunit at C-terminal residues Ser-1901 and Tyr-2122. Likewise, BK channel potentiation by α5β1 integrin activation requires c-Src phosphorylation of the channel α-subunit at residue Tyr-766. Thus, both L-type calcium channels and BK channels can be regulated coordinately through integrin-linked phosphorylation cascades involving c-Src. We propose that these two channels are under constitutive control by α5β1 integrin-fibronectin interactions in the vessel wall such that the balance of their activity determines myogenic tone and the vascular response to vessel wall injury/remodeling.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Vuori K. Integrin signaling: tyrosine phosphorylation events in focal adhesions. J Membr Biol 1998; 165(3):191–9.
Larsen M, Artym VV, Green JA et al. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol 2006; 18(5):463–71.
Schwartz MA. Integrin signaling revisited. Trends Cell Biol 2001; 11:466–470.
Giancotti FG, Ruoslahti E. Integrin signaling. Science 1999; 285:1028–1032.
Ingber DE. Cellular mechanotransduction: putting all the pieces together. FASEB J 2006; 20(7):811–27.
Gao M, Sotomayor M, Villa E et al. Molecular mechanisms of cellular mechanics. Phys Chem Chem Phys 2006; 8(32):3692–706.
Glukhova MA, Koteliansky VE. Integrins, cytoskeletal and extracellular matrix proteins in developing smooth muscle cells of human aorta. In: Schwartz SM and Mecham RP, eds. The Vascular Smooth Muscle Cell: Molecular and Biological Responses to the Extracellular Matrix. San Diego, CA: Academic 1995; 37–79.
Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech 2005; 38(10):1949–71.
Muller JM, Chilian WM, Davis MJ. Integrin signaling transduces shear stress-dependent vasodilation of coronary arterioles. Circ Res 1997; 80:320–326.
Madden JA, Christman NJ. Integrin signaling, free radicals and tyrosine kinase mediate flow constriction in isolated cerebral arteries. Am J Physiol (Heart Circ Physiol) 1999; 277:H2264–H2271.
Mogford JE, Davis GE, Platts SH et al. Vascular smooth muscle αvβ3 integrin mediates arteriolar vasodilation in response to RGD peptides. Circ Res 1996; 79:821–826.
Mogford JE, Davis GE, Meininger GA. RGDN peptide interaction with endothelial α5β1 integrin causes sustained endothelin-dependent vasoconstriction of rat skeletal muscle arterioles. J Clin Invest 1997; 100:1647–1653.
Lipke DW, Soltis EE, Fiscus RR et al. RGD-containing peptides induce endothelium-dependent and independent vasorelaxations of rat aortic rings. Regul Pept 1996; 63:23–29.
Yip KP, Marsh DJ. An Arg-Gly-Asp peptide stimulates constriction in rat afferent arteriole. Am J Physiol (Renal Physiol) 1997; 273:F768–F776.
Laplante C, St-Pierre S, Beaulieu AD et al. Small fibronectin fragments induce endothelium-dependent vascular relaxations. Can J Physiol Pharmacol 1988; 66:745–748.
Martinez-Lemus LA, Crow T, Davis MJ et al. αvβ3-and α5β1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol (Heart Circ Physiol) 2005; 289:H322–9.
Waitkus-Edwards KR, Martinez-Lemus LA, Wu X et al. α4β1Integrin activation of L-type calcium channels in vascular smooth muscle causes arteriole vasoconstriction. Circ Res 2002; 90(4):473–80.
Platts, SH, Mogford JE, Davis MJ et al. Role of K+ channels in arteriolar vasodilation mediated by integrin interaction with RGD-containing peptide. Am J Physiol (Heart Circ Physiol) 1998; 275:H1449–H1454.
Ledoux J, Werner ME, Brayden JE et al. Calcium-activated potassium channels and the regulation of vascular tone. Physiology 2006; 21:69–78.
Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 1999; 79(2):387–423.
D’Angelo, G, Mogford JE, Davis GE et al. Integrin-mediated reduction in vascular smooth muscle [Ca2+]i induced by RGD-containing peptide. Am J Physiol (Heart Circ Physiol) 1999; 272:H2065–H2070.
Wu, X, Mogford JE, Platts SH et al. Modulation of calcium current in arteriolar smooth muscle by αvβ3 and α5β1 integrin ligands. J Cell Biol 1998; 143:241–252.
Miyamoto S, Akiyama SK, Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science 1995; 267:883–885.
Wu X, Davis GE, Meininger GA et al. Regulation of the L-type calcium channel by α5β1 integrin requires signaling between focal adhesion proteins. J Biol Chem 2001; 276:30285–30292.
Wijetunge S, Hughes AD. pp60c-src increases voltage-operated calcium channel currents in vascular smooth muscle cells. Biochem Biophys Res Comm 1995; 217:1039–1044.
Wijetunge S, Hughes AD. Activation of endogenous c-Src or a related tyrosine kinase by intracellular (pY)EEI peptide increases voltage-operated calcium channel currents in rabbit ear artery cells. FEBS Lett 1996; 399:63–66.
Hu XQ, Singh N, Mukhopadhyay D et al. Modulation of voltage-dependent Ca2+ channels in rabbit colonic smooth muscle cells by c-Src and focal adhesion kinase. J Biol Chem 1998; 273:5337–5342.
Wijetunge S, Hughes AD. Effect of platelet-derived growth factor on voltage-operated calcium channels in rabbit isolated ear artery cells. Br J Pharmacol 1995; 115:534–538.
Gui P, Balasubramanian BB, Wu X et al. Effect of α5β1 integrin activation on heterogenously expressed cardiac L-type calcium channel. Biophysical J 2004; 86(1) 427A.
Wang YG, Samarel AM, Lipsius SL. Laminin acts via β1 integrin signalling to alter cholinergic regulation of L-type Ca(2+) current in cat atrial myocytes. J Physiol 2000; 526(1):57–68.
Wang YG, Samarel AM, Lipsius SL. Laminin binding to β1-integrins selectively alters beta1-and beta2-adrenoceptor signalling in cat atrial myocytes. J Physiol 2000; 527(1):3–9.
Gui P, Wu X, Ling S et al. Integrin receptor activation triggers converging regulation of Cav1.2 calcium channels by c-Src and protein kinase A pathways. J Biol Chem 2006; 281(20):14015–25.
Chao J-T, Gui P, Zamponi G et al. Spatial association between L-type calcium channels and integrins. FASEB J 2007; 21(5):A914.
Lin CY, Hilgenberg LG, Smith MA et al. Integrin regulation of cytoplasmic calcium in excitatory neurons depends upon glutamate receptors and release from intracellular stores. Mol Cell Neurosci 2008; 37(4):770–80.
Gall CM, Pinkstaff JK, Lauterborn JC et al. Integrins regulate neuronal neurotrophin gene expression through effects on voltage-sensitive calcium channels. Neuroscience 2003; 118(4):925–40.
Stäubli U, Chun D, Lynch G. Time-dependent reversal of long-term potentiation by an integrin antagonist. J Neurosci 1998; 18(9):3460–9.
Eliceiri BP. Integrin and growth factor receptor crosstalk. Circ Res 2001; 89(12):1104–10.
Bryan RM Jr, Marrelli SP, Steenberg ML et al. Effects of luminal shear stress on cerebral arteries and arterioles. Am J Physiol (Heart Circ Physiol) 2001; 280(5):H2011–22.
Leavesley DI, Schwartz MA, Rosenfeld M et al. Integrin β1-and β3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol 1993; 121(1):163–70.
Bhattacharya S, Ying X, Fu C et al. αvβ3 integrin induces tyrosine phosphorylation-dependent Ca2+ influx in pulmonary endothelial cells. Circ Res 2000; 86(4):456–62.
Kawasaki J, Davis GE, Davis MJ. Regulation of Ca2+-dependent K+ current by αvβ3 integrin engagement in vascular endothelium. J Biol Chem 2004; 279(13):12959–66.
Daut J, Mehrke G, Nees S et al. Passive electrical properties and electrogenic sodium transport of cultured guinea-pig coronary endothelial cells. J Physiol 1988; 402:237–54.
Peppelenbosch MP, Tertoolen LG, de Laat SW. Epidermal growth factor-activated calcium and potassium channels. J Biol Chem 1991; 266(30):19938–44.
Sharma NR, Davis MJ. Mechanism of substance P-induced hyperpolarization of porcine coronary artery endothelial cells. Am J Physiol (Heart Circ Physiol) 1994; 266:H156–64.
Vaca L, Licea A, Possani LD. Modulation of cell membrane potential in cultured vascular endothelium. Am J Physiol (Cell Physiol) 1996; 270:C819–24.
Hofmann G, Bernabei PA, Crociani O et al. HERG K+ channels activation during β1 integrin-mediated adhesion to fibronectin induces an up-regulation of αvβ3 integrin in the preosteoclastic leukemia cell line FLG 29.1. J Biol Chem 2001; 276(7):4923–31.
Cherubini A, Hofmann G, Pillozzi S et al. Human ether-a-go-go-related gene 1 channels are physically linked to β1 integrins and modulate adhesion-dependent signaling. Mol Biol Cell 2005; 16(6):2972–83.
Becchetti A, Arcangeli A, Del Bene MR et al. Response to fibronectin-integrin interaction in leukaemia cells: delayed enhancing of a K+ current. Proc Biol Sci 1992; 248(1323):235–40.
Wu X, Yang Y, Gui P et al. Potentiation of BK channels by α5β1 integrin activation in arteriolar smooth muscle. J Physiol 2008; 586:1699–1713.
Yang Y, Wu X, Wu J et al. Integrin activation results in c-Src mediated phosphorylation and potentiation of Maxi K channels. FASEB J 2007; 21(5):A538.
Lal H, Guleria RS, Foster DM et al. Integrins: novel therapeutic targets for cardiovascular diseases. Cardiovasc Hematol Agents Med Chem 2007; 5(2):109–32.
Hayden MR, Sowers JR, Tyagi SC. The central role of vascular extracellular matrix and basement membrane remodeling in metabolic syndrome and type 2 diabetes: the matrix preloaded. Cardiovasc Diabetol 2005; 4(1):9.
del Zoppo GJ, Milner R. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol 2006; 26(9):1966–75.
Heerkens EH, Izzard AS, Heagerty AM. Integrins, vascular remodeling and hypertension. Hypertension 2007; 49(1):1–4.
Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol 2007; 27(11):2302–9.
Intengan HD, Schiffrin EL. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension 2000; 36(3):312–8.
Hultgårdh-Nilsson A, Durbeej M. Role of the extracellular matrix and its receptors in smooth muscle cell function: implications in vascular development and disease. Curr Opin Lipidol 2007; 18(5):540–5.
Davis GE, Bayless KJ, Davis MJ et al. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol 2000; 156:1489–1498.
Davis MJ, Wu X, Nurkiewicz T et al. Regulation of ion channels by integrins. Cell Biochemistry and Biophysics 2002; 36(1):41–66.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Landes Bioscience and Springer Science+Business Media
About this chapter
Cite this chapter
Gui, P., Chao, JT., Wu, X., Yang, Y., Davis, G.E., Davis, M.J. (2010). Coordinated Regulation of Vascular Ca2+ and K+ Channels by Integrin Signaling. In: Becchetti, A., Arcangeli, A. (eds) Integrins and Ion Channels. Advances in Experimental Medicine and Biology, vol 674. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-6066-5_7
Download citation
DOI: https://doi.org/10.1007/978-1-4419-6066-5_7
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-6065-8
Online ISBN: 978-1-4419-6066-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)