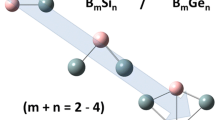
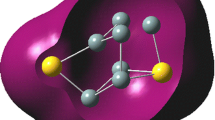
Abstract
Chemistry of polyhedral silicon and germanium clusters is summarized. Historical background, synthesis, structure, physical properties, and reactions are described in detail.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- t-Bu:
-
Tert-Butyl
- 18-Cr-6:
-
18-Crown-6
- cat:
-
Catalyst
- d:
-
Day(s)
- DEP:
-
2,6-Diethyphenyl
- DME:
-
1,2-Dimethoxyethane
- DMF:
-
Dimethylformamide
- DMSO:
-
Dimethyl sulfoxide
- equiv:
-
Equivalent(s)
- e.g.:
-
For example
- Et:
-
Ethyl
- exc.:
-
Excess
- h:
-
Hour(s)
- HMPA:
-
Hexamethylphosphoric triamide
- LiNapht:
-
Lithium naphthalenide
- m-CPBA:
-
m-Chloroperoxybenzoic acid
- Me:
-
Methyl
- Mes:
-
Mesityl, 2,4,6-trimethylphenyl
- MS:
-
Mass spectrometory
- min:
-
Minute(s)
- mol:
-
Mole(s)
- NBS:
-
N-Bromosuccinimide
- NCS:
-
N-Chlorosuccinimide
- Nu:
-
Nucleophile
- Ph:
-
Phenyl
- Pr:
-
Propyl
- i-Pr:
-
Isopropyl
- py:
-
Pyridine
- rt:
-
Room temperature
- s:
-
Second(s)
- SCE:
-
Saturated calomel electrode
- TBAF:
-
Tetrabutylammonium fluoride
- TBDMS:
-
Tert-butyldimethylsilyl
- TBDPS:
-
Tert-butyldiphenylsilyl
- Tf:
-
Trifluoromethanesulfonyl (triflyl)
- TFA:
-
Trifluoroacetic acid
- TGA:
-
Thermogravimetric
- thexyl:
-
1,1,2-Trimethylpropyl
- THF:
-
Tetrahydrofuran
- TEP:
-
2,4,6-Triethylphenyl
- TIP:
-
2,4,6-Triisopropylphenyl
- TIPS:
-
Triisopropylsilyl
- TMEDA:
-
N,N,N′,N′-tetramethyl-1,2-ethylenediamine
- TMS:
-
Trimethylsilyl
- Tol:
-
4-Methylphenyl
- trityl:
-
Triphenylmethyl
References
Greenberg A, Liebman JF (1978) Strained organic molecules. Academic, New York
Balaban AT, Banciu M, Ciorba V (1987) Annulenes, benzo-, hetero-, homo-derivatives, and their valence isomers. CRC, Florida
Matsumoto H, Higuchi K, Hoshino Y, Koike H, Naoi Y, Nagai Y (1988) The first octasilacubane system: synthesis of octakis-(t-butyldimethylsilyl)pentacyclo[4.2.0.02,5.03,8.04,7]octasilane. J Chem Soc, Chem Commun :1083
Sekiguchi A, Nagase S (1998) Polyhedral silicon compounds. In: Rappoport Z, Apeloig Y (eds) The chemistry of organosilicon compounds, vol 2. Wiley, West Sussex
Unno M, Matsumoto T, Mochizuki K, Higuchi K, Goto M, Matsumoto H (2003) Structure and oxidation of octakis(tert-butyldimethylsilyl)octasilacubane. J Organomet Chem 685:156
Kabe Y, Kuroda M, Honda Y, Yamashita O, Kawase T, Masamune S (1988) Reductive oligomerization of 1,2-di-tert-butyl-1,1,2,2-tetrachlorodisilane: the tricyclo[2.2.0.02,5]hexasilane and tetracyclo[3.3.0.02,7.03,6] octasilane systems. Angew Chem Int Ed 27:1725
Weidenbruch M, Grimm F-T, Pohl S, Saak W (1989) A polyhedral oligogermane: 4,8-dibromoocta-tert-butyltetracyclo [3.3.0.02,7.03,6] octagermane. Angew Chem Int Ed 28:198
Sekiguchi A, Naito H, Nameki H, Ebata K, Kabuto C, Sakurai H (1989) 4,8-dichloroocta-t-butyltetracyclo[3.3.0.02,7.03,6]octagermane. J Organomet Chem 368:C1
Sekiguchi A, Yatabe T, Kamatani H, Kabuto C, Sakurai H (1992) Chemistry of organosilicon compounds. 293. Preparation, characterization, and crystal structures of octasilacubanes and octagermacubanes. J Am Chem Soc 114:6260
Matsumoto H, Higuchi K, Kyushin S, Goto M (1992) Octakis(1,1,2-trimethylpropyl)octasilacubane: synthesis, molecular structure, and unusual properties. Angew Chem Int Ed 31:1354
Furukawa K, Fujino M, Matsumoto N (1992) Cubic silicon cluster. Appl Phys Lett 60:2744
Unno M, Higuchi K, Furuya K, Shioyama H, Kyushin S, Goto M, Matsumoto H (2000) Synthesis, structure, and reactions of octakis(1,1,2-trimethylpropyl)octagermacubane. Bull Chem Soc Jpn 73:2093
Sekiguchi A, Sakurai H (1995) Cage and cluster compounds of silicon, germanium, and tin. In: Stone FGA, West R (eds) Advances in organometallic chemistry, vol 37. Academic, San Diego
Nagase S (1993) Theoretical study of heteroatom-containing compounds. From aromatic and polycyclic molecules to hollow cage clusters. Pure Appl Chem 65:675
Sekiguchi A, Kabuto C, Sakurai H (1989) [(Me3Si)2CHGe]6, the first hexagermaprismane. Angew Chem Int Ed 28:55
Sekiguchi A, Yatabe T, Kabuto C, Sakurai H (1993) Chemistry of organosilicon compounds. 303. The missing hexasilaprismane: synthesis, x-ray analysis and photochemical reactions. J Am Chem Soc 115:5853
Abersfelder K, Russell A, Rzepa HS, White AJP, Peter Haycock PR, Scheschkewitz D (2012) Contraction and expansion of the silicon scaffold of stable Si6R6 isomers. J Am Chem Soc 134:16008
Nagase S (1989) Much less strained cubane analogues with Si, Ge, Sn, and Pb skeletons. Angew Chem Int Ed 28:329
Wiberg N, Finger CMM, Polborn K (1993) Tetrakis(tri-tert-butylsilyl)-tetrahedro-tetrasilane (tBu3Si)4Si4: the first molecular silicon compound with a Si4 tetrahedron. Angew Chem Int Ed 32:1054
Ichinohe M, Toyoshima M, Kinjo R, Sekiguchi A (2003) Tetrasilatetrahedranide: a silicon cage anion. J Am Chem Soc 125:13328
Meyer-Wegner F, Scholz S, Sänger I, Schodel F, Bolte M, Wagner M, Lerner H-W (2009) Synthesis of Wiberg’s tetrasilatetrahedrane (tBu3Si)4Si4 by a one-pot procedure. Organometallics 28:6835
Cordes DB, Lickiss PD, Rataboul F (2010) Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem Rev 110:2081
Unno M, Yokota T, Matsumoto H (1996) Oxaoctasilahomocubane and dioxaoctasilabishomocubane: novel silicon ring system. J Organomet Chem 521:409
Unno M, Yamashita N, Matsumoto H (2011) Thermal reaction of octasilacubane with sulfur, selenium, and tellurium: formation of novel cage systems. Phosphorus, Sulfur, Silicon and Relat Elem 186:1259
Unno M, Higuchi K, Ida M, Shioyama H, Kyushin S, Goto M, Matsumoto H (1994) Ring-opening reaction of octakis(1,1,2-trimethylpropyl)octasilacubane. Organometallics 13:4633
Unno M, Masuda H, Matsumoto H (2002) Photo-initiated bromination of octakis(1,1,2-trimethylpropyl)octasilacubane with tetrabromomethane. Silicon Chem 1:377
Unno M, Shioyama H, Ida M, Matsumoto H (1995) Reductive dehalogenation of 4,8-dihalooctakis(1,1,2-trimethylpropyl)tetracyclo-[3.3.0.02,7.03,6]octasilanes with sodium. Organometallics 14:4004
Horiuchi H, Nakano Y, Matsumoto T, Unno M, Matsumoto H, Hiratsuka H (2000) Electronic structure and photochemical reaction intermediates of octakis(1,1,2-trimethylpropyl)octasilacubane. Chem Phys Lett 322:33
Kyushin S, Meguro A, Unno M, Matsumoto H (2000) Photolysis of anti-dodecaalkyltricyclo[4.2.0.02,5]octasilane: generation and reactions of cyclotetrasilene. Chem Lett 29:494
Wiberg N, Auer H, Wagner S, Polborn K, Kramer G (2001) Disilene R*XSi=SiXR* (R* = SitBu3) mit siliciumgebundenen H- und Hal-atomen X: bildung, isomerisierung, reaktionen. J Organomet Chem 619:110
Wiberg N, Auer H, Noth H, Knizek J, Polborn K (1998) Diiodotetrasupersilylcyclotetrasilene (tBu3Si)4Si4I2 – a molecule containing an unsaturated Si4 ring. Angew Chem Int Ed 37:2869
Ichinohe M, Takahashi N, Sekiguchi A (1999) Formation and structure of protonated tetrasilatetrahedrane-monooxide, (tert-Bu3Si)4Si4OH. Chem Lett 28:553
Wiberg N, Auer H, Polborn K, Veith M, Huch V (2000) Products of the reaction of tetrasupersilyl-tetrahedro-tetrasilane (tBu3Si)4Si4 with iodine. In: Auner N, Weis J (eds) Organosilicon chemistry IV – from molecules to material. Wiley-VCH, Weinheim
Fischer G, Huch V, Mayer P, Vasisht SK, Veith M, Wiberg N (2005) Si8(SitBu3)6: A hitherto unknown cluster structure in silicon chemistry. Angew Chem Int Ed 44:7884
Wiberg N, Vasisht SK, Fischer G, Mayer P, Huch V, Veith M (2003) Reactivity of the unusually structured silicon cluster compound Si8(SitBu3)6. Z Anorg Allg Chem 633:2425
Klapötke TM, Vasisht SK, Fischer G, Mayer P (2010) A reactive Si4 cage: K(SitBu3)3Si4. J Organomet Chem 695:667
Klapötke TM, Vasisht SK, Mayer P (2010) Spirocycle (SitBu3)6Si9Cl2: the first of its kind among group 14 elements. Eur J Inorg Chem :3256
Iwamoto T, Tsushima D, Kwon E, Ishida S, Isobe H (2012) Persilastaffanes: design, synthesis, structure, and conjugation between silicon cages. Angew Chem Int Ed 51:2340
Kabe Y, Kawase T, Okada J, Yamashita O, Goto M, Masamune S (1990) A bicyclo[1.1.1]pentasilane derivative. Synthesis, molecular structure, and comments on structural homology. Angew Chem Int Ed 29:794
Augenstein T, Oña-Burgos P, Nied D, Breher F (2012) Alkynyl-functionalised and linked bicyclo[1.1.1]pentanes of group 14. Chem Commun 48:6803
Tsumuraya T, Batcheller SA, Masamune S (1991) Strained-ring and double-bond systems consisting of the Group 14 elements Si, Ge, and Sn. Angew Chem Int Ed 30:902
Sita LR, Bickerstaff RDJ (1989) 2,2,4,4,5,5-Hexakis(2,6-diethylphenyl)pentastanna[1.1.1]propellane: characterization and molecular structure. J Am Chem Soc 111:6454
Nied D, Köppe R, Klopper W, Schnöckel H, Breher F (2010) Synthesis of a pentasilapropellane. Exploring the nature of a stretched silicon–silicon bond in a nonclassical molecule. J Am Chem Soc 132:10264
Wiberg N, Schuster H, Simon A, Peters K (1986) Hexa-tert-butyldisilane–the molecule with the longest Si–Si bond. Angew Chem Int Ed 25:79
Nied D, Klopper W, Breher F (2009) Pentagerma[1.1.1]propellane: a combined experimental and quantum chemical study on the nature of the interactions between the bridgehead atoms. Angew Chem Int Ed 48:1411
Lee VA, Ichinohe M, Sekiguchi A (2002) 2,4-Disila-1-germatricyclo[2.1.0.02,5]pentane: a new type of cage compound of group 14 elements with an extremely long Ge–C bridge bond and an “umbrella”-type configuration of a Ge atom. J Am Chem Soc 124:9962
Lee VA, Yokoyama T, Takanishi K, Sekiguchi A (2009) Pentasilatricyclo[2.1.0.02,5]pentane and its anion. Chem Eur J 15:8401
Takahashi N, Lee VY, Ichinohe M, Sekiguchi A (2007) 1,2,5,6-Tetrasilabenzobenzvalene: a valence isomer of 1,2,3,4-tetrasilanaphthalene. Chem Lett 36:1158
Scheschkewitz D (2004) A silicon analogue of vinyllithium: structural characterization of a disilenide. Angew Chem Int Ed 43:2965
Abersfelder K, White AJP, Rzepa HS, Scheschkewitz D (2010) A tricyclic aromatic isomer of hexasilabenzene. Science 327:564
Abersfelder K, White AJP, Berger RJF, Rzepa HS, Scheschkewitz D (2011) A stable derivative of the global minimum on the Si6H6 potential energy surface. Angew Chem Int Ed 50:7936
Schnepf A, Köppe R R (2003) Ge8{N(SiMe3)2}6: a ligand-stabilized Ge cluster compound with formally zero-valent Ge atoms. Angew Chem Int Ed 42:911
Richards AF, Hope H, Power PP (2003) Synthesis and characterization of neutral, homo and heteronuclear clusters with unsubstituted germanium or tin atoms. Angew Chem Int Ed 42:4071
Fischer J, Baumgartner J, Marschner C (2005) Synthesis and structure of sila-adamantane. Science 310:825
Fischer R, Konopa T, Ully S, Baumgartner J, Marschner C C (2003) Route Si6 revisited. J Organomet Chem 685:79
West R, Indriksons A (1972) Cyclic polysilanes. VI. Bicyclic and cage permethylcyclopolysilanes. J Am Chem Soc 94:6110
Ishida S, Otsuka K, Toma Y, Kyushin S (2013) An organosilicon cluster with an octasilacuneane core: a missing silicon cage motif. Angew Chem 52:2507
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Unno, M. (2013). Substituted Polyhedral Silicon and Germanium Clusters. In: Scheschkewitz, D. (eds) Functional Molecular Silicon Compounds II. Structure and Bonding, vol 156. Springer, Cham. https://doi.org/10.1007/430_2013_99
Download citation
DOI: https://doi.org/10.1007/430_2013_99
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-03733-2
Online ISBN: 978-3-319-03734-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)