Abstract
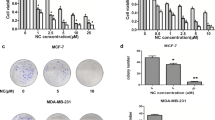
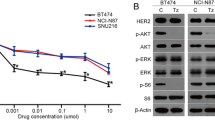
HER2 expression is associated with 30% of breast cancer patients with a poor prognosis. Though Trastuzumab is approved for HER2 targeted therapy, its use is limited because of its systemic toxicity and resistance in most patients. This study evaluated the synergistic effects of Phenethyl isothiocyanate (PEITC) and Curcumin (CUR) in HER2 overexpressing SK-BR-3, BT-474, and AU-565 breast cancer cells. The cytotoxic effect of PEITC : CUR against breast cancer cells was evaluated using an MTT assay, and the Loewe additivity model was used to evaluate the synergistic effect. Apoptosis induction and cell cycle arrest over the treatment of PEITC: CUR in breast cancer cells were examined using the flow cytometric annexin-V/Propidium iodide method. Downregulation of HER2-mediated signaling was deduced from protein expression analysis using western-blot. Our results showed that treatment of PEITC : CUR at varying levels of combinations in all three breast cancer cells extensively reduced the survival of the cells with the lowest inhibitory concentrations (IC50). Cytotoxic data revealed that the 3 : 1 ratio of PEITC : CUR was the best among several (1 : 1, 3 : 1, and 1 : 3) combinations, with the maximum cytotoxicity. PEITC : CUR (3 : 1) displayed the lowest combination index (CI) against SK-BR-3, and AU-565 cells indicated its potential synergistic effect. At twice the concentration of its IC50, the 3 : 1 combination elicited 3.5 to 4.5 fold apoptosis in HER2 overexpressing cells, approximately double the effect of the individual drugs alone. In addition, the selected combination induced the G2/M cell cycle arrest in HER2 expressing cells over the treatment. Western blot protein expression analysis revealed that the PEITC : CUR combination suppressed the HER2/PI3K/Akt signaling, eventually connected to various apoptotic biological events. Our results showed the specificity of PEITC: CUR combination in inducing apoptosis and G2/M cell cycle arrest in HER2-expressing tumor cells in-vitro and enhancing the anti-cancer effect. For a subset of breast cancer patients who overexpress HER2, this combination of PEITC and CUR could be a potential treatment option.







Similar content being viewed by others
Change history
31 January 2024
Modifications have been made to the Publisher’s Note.
REFERENCES
Aggarwal, B.B., Kumar, A., and Bharti, A.C., et al., Anticancer potential of curcumin: Preclinical and clinical studies, Anticancer Res., 2003, vol. 23, pp. 363–398. https://doi.org/10.23122a:363-98
Agrawal, S., Late effects of cancer treatment in breast cancer survivors, S. Asian J. Cancer, 2014, vol. 3, no. 2, pp. 112–115. https://doi.org/10.4103/2278-330X.130445
Aman, N.A., Doukoure, B., Koffi, K.D., et al., HER2 overexpression and correlation with other significant clinicopathologic parameters in Ivorian breast cancer women, BMC Clin. Pathol., 2019, vol. 19, p. 1. https://doi.org/10.1186/s12907-018-0081-4
Boreddy, S.R. and Srivastava, S.K., Deguelin suppresses pancreatic tumor growth and metastasis by inhibiting epithelial-to-mesenchymal transition in an orthotopic model, Oncogene, 2013, vol. 32, no. 34, pp. 3980–3991. https://doi.org/10.1038/onc.2012.413
Cang, S., Ma, Y., Chiao, J.W., Liu, D., et al., Phenethyl isothiocyanate and paclitaxel synergistically enhanced apoptosis and alpha-tubulin hyperacetylation in breast cancer cells, Exp. Hematol. Oncol., 2014, vol. 3, pp. 5–13. https://doi.org/10.1186/2162-3619-3-5
Cavell, B.E., Syed Alwi, S.S., Donlevy, A.M., Proud, C.G., Packham, G., et al., Natural product-derived anti-tumor compound phenethyl isothiocyanate inhibits mTORC1 activity via TSC2, J. Nat. Prod., 2012, vol. 75, pp. 1051–1057. https://doi.org/10.1021/np300049b
Daniela, B., Cinzia, G., Francesca, D.A., Marilena, L., Sebastiano, A., et al., Natural products as promising antitumoral agents in breast cancer: mechanisms of action and molecular targets, Mini-Rev. Med. Chem., 2016, vol. 16, no. 8, pp. 596–604. https://doi.org/10.2174/1389557515666150709110959
Deveraux, Q.L., Takahashi, R., Salvesen, G.S., Reed, J.C., et al., X-linked IAP is a direct inhibitor of cell-death proteases, Nature, 1997, vol. 388, pp. 300–304. https://doi.org/10.1038/40901
Dubrez, D.L., Dupoux, A., Cartier, J., et al., IAPs: More than just inhibitors of apoptosis proteins, Cell Cycle, 2008, vol. 7, pp. 1036–1046. https://doi.org/10.4161/cc.7.8.5783
Fadus, M.C., Lau, C., Bikhchandani, J., Lynch, H.T., et al., Curcumin: An age-old anti-inflammatory and anti-neoplastic agent, J. Tradit. Complementary Med., 2016, vol. 7, pp. 339–346. https://doi.org/10.1016/j.jtcme.2016.08.002
Gong, A., He, M., Krishna, V.D., Yin, P., Karnes, R.J., Young, C.Y., et al., Phenethyl isothiocyanate inhibits STAT3 activation in prostate cancer cells, Mol. Nutr. Food. Res., 2009, vol. 53, no. 7, pp. 878–886. https://doi.org/10.1002/mnfr.200800253
Grogan, F. and Cobain, E.F., Breast cancer management in 2021: A primer for the obstetrics and gynecology, Best Pract. Res. Clin. Obstet. Gynaecol., 2022, vol. 26, no. 8, pp. 1521–1534. https://doi.org/10.1016/j.bpobgyn.2022.02.004
Han, X., Deng, S., Wang, N., Liu, Y., and Yang, X., et al., Inhibitory effects and molecular mechanisms of tetrahydrocurcumin against human breast cancer MCF-7 cells, Food. Nutr. Res., 2016, vol. 60, pp. 3061–3066. https://doi.org/10.3402/fnr.v60.30616
Harbeck, N., Penault, L.F., Cortes, J., Michael, G., Nehmat, H., Philip, P., et al., Breast cancer, Nat. Rev. Dis. Primers, 2019, vol. 5, no. 1, p. 66. https://doi.org/10.1038/s41572-019-0111-2
Henjes, F., Bender, C., Vonder, H.S., Braun, L., Mannsperger, H.A., Schmidt, C., et al., Strong EGFR signaling in cell line models of ERBB2-amplified breast cancer attenuates response towards ERBB2-targeting drugs, Oncogenesis, 2012, vol. 2, pp. 1–16. https://doi.org/10.1038/oncsis.2012.16
Hu, A., Huang, J.J., Zhang, J.F., et al., Curcumin induces G2/M cell cycle arrest and apoptosis of head and neck squamous cell carcinoma in vitro and in vivo through ATM/Chk2/p53-dependent pathway, Oncotarget, 2017, vol. 8, no. 31, pp. 50747–50760. https://doi.org/10.18632/oncotarget.17096
Hudis, C.A., Trastuzumab mechanism of action and use in clinical practice, N. Engl. J. Med., 2007, vol. 357, no. 1, pp. 39–51. https://doi.org/10.1056/NEJMra043186
Karunagaran, D., Rashmi, R., Kumar, T.R., et al., induction of apoptosis by curcumin and its implications for cancer therapy, Curr. Cancer Drug Targets, 2005, vol. 5, no. 2, pp. 117–129. https://doi.org/10.2174/1568009053202081
Lai, H.W., Chien, S.Y., Kuo, S.J., Ling, M.T., Hui, Y.L., and Chin, W.C., The potential utility of curcumin in the treatment of HER-2-overexpressed breast cancer: An in vitro and in vivo comparison study with herceptin, J. Evidence-Based Integr. Med., 2012, vol. 12, pp. 486–493. https://doi.org/10.1155/2012/486568
Lederer, S., Dijkstra, T., Heskes, T., et al., Additive dose-response models: explicit formulation and the loewe additivity consistency condition, Front. Pharmacol., 2018, vol. 9, pp. 31–39. https://doi.org/10.3389/fphar.2018.00031
Martínez, C.M., Villegas, S.N., Meraz-Rios, M.A., et al., Curcumin differentially affects cell cycle and cell death in acute and chronic myeloid leukemia cells, Oncol. Lett., 2018, vol. 15, no. 5, pp. 6777–6783. https://doi.org/10.3892/ol.2018.8112
Mashati, P., Esmaeili, S., Dehghan, N., Bashash, D., Darvishi, M., Gharehbaghian, A., et al., Methanolic extract from aerial parts of artemisia annua l. induces cytotoxicity and enhances vincristine-induced anticancer effect in Pre-b acute lymphoblastic leukemia cells, Int. J. Hematol. Oncol. Stem Cell Res., 2019, vol. 13, no. 3, pp. 132–139. https://doi.org/2019-Jul-1-13(3):132-139
Meric, B.F. and Hung, M.C., Advances in targeting human epidermal growth factor receptor-2 signaling for cancer therapy, Clin. Cancer Res., 2006, vol. 12, no. 21, pp. 6326–6330. https://doi.org/10.3810/hp.2012.10.997
Moon, Y.J., Brazeau, D.A., Morris, M.E., et al., Dietary phenethyl isothiocyanate alters gene expression in human breast cancer cells, J. Evidence-Based Integr. Med., 2011, vol. 20, no. 1, pp. 46251–46258. https://doi.org/10.1155/2011/462525
Oh, D. and Bang, Y., HER2-targeted therapies – a role beyond breast cancer, Nat. Rev. Clin. Oncol., 2020, vol. 17, pp. 33–48. https://doi.org/10.1038/s41571-019-0268-3
Ortega, M.A., Fraile, M.O., Asúnsolo, Á., Buján, J., García, H.N., Coca, S., et al., Signal transduction pathways in breast cancer: The important role of PI3K/Akt/mTOR, J. Oncol., 2020, vol. 2020, p. 9258396. https://doi.org/10.1155/2020/9258396
Park, J.E., Sun, Y., Lim, S.K., et al., Dietary phytochemical PEITC restricts tumor development via modulation of epigenetic writers and erasers, Sci. Rep., 2017, vol. 7, p. 40569. https://doi.org/10.1038/srep40569
Rowe, D.L., Ozbay, T., Regan, R.M., Rita, N., et al., Modulation of the BRCA1 protein and induction of apoptosis in triple-negative breast cancer cell lines by the polyphenolic compound curcumin, Breast Cancer: Basic Clin. Res., 2009, vol. 3, pp. 61–75. https://doi.org/10.4137/bcbcr.s3067
Sarkars, R., Mukherjee, S., Roy, M., et al., Targeting heat shock proteins by phenethyl isothiocyanate results in cell-cycle arrest and apoptosis of human breast cancer cells, Nutr. Cancer, 2013, vol. 65, pp. 480–493. https://doi.org/10.1080/01635581.2013.767366
Shao, Z.M., Shen, Z.Z., Liu, C.H., Maryam, R., Vay, L.G., David, H., et al., Curcumin exerts multiple suppressive effects on human breast carcinoma cells, Int. J. Cancer, 2002, vol. 98, pp. 234–240. https://doi.org/10.1002/ijc.10183
Shishodia, S., Sethi, G., Aggarwal, B.B., et al., Curcumin: getting back to the roots, Ann. N. Y. Acad. Sci., 2005, vol. 1056, pp. 206–217. https://doi.org/10.1196/annals.1352.010
Shoaib, S., Tufail, S., Sherwani, M.A., et al., Phenethyl isothiocyanate induces apoptosis through ROS generation and caspase-3 activation in cervical cancer cells, Front. Pharmacol., 2021, vol. 12, no. 3, pp. 673–682. https://doi.org/10.3389/fphar.2021.673103
Singh, A.V., Xiao, D., Lew, K.L., Dhir, R., Singh, S.V., et al., Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo, Carcinogenesis, 2004, vol. 25, pp. 83–90. https://doi.org/10.1093/carcin/bgg178
Slamon, D.J., Clark, G.M., Wong, S.G., et al., Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene, Science, 1987, vol. 235, no. 4785, pp. 177–182. https://doi.org/10.1126/science.3798106
Slamon, D.J., Godolphin, W., Jones, L.A., Holt, J.A., Wong, S.G., Keith, D.E., et al., Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer, Science, 1989, vol. 244, no. 4905, pp. 707–712. https://doi.org/10.1126/science.2470152
Subik, K., Lee, J.F., Baxter, L., Tamera, S., Dawn, C., Patti, C., et al., The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by immunohistochemical analysis in breast cancer cell lines, Breast Can., 2010, vol. 4, pp. 35–41. https://doi.org/10.1177/1178223418806626
Syed Alwi, S.S., Cavell, B.E., Donlevy, A., Packham, G., et al., Differential induction of apoptosis in human breast cancer cell lines by phenethyl isothiocyanate, a glutathione depleting agent, Cell Stress Chaperones, 2012, vol. 17, pp. 529–538. https://doi.org/10.1007/s12192-012-0329-3
Upadhyaya, B., Liu, Y., and Dey, M., Phenethyl isothiocyanate exposure promotes oxidative stress and suppresses Sp1 transcription factor in cancer stem cells, Int. J. Mol. Sci., 2019, vol. 20, no. 5, pp. 1027–1034. https://doi.org/10.3390/ijms20051027
Wang, J. and Xu, B., Targeted therapeutic options and future perspectives for HER2-positive breast cancer, Signal Transduction Targeted Ther., 2019, vol. 4, p. 34. https://doi.org/10.1038/s41392-019-0069-2
Xiao, D., Zeng, Y., Choi, S., Lew, K.L., Nelson, J.B., Singh, S.V., et al., Caspase-dependent apoptosis induction by phenethyl isothiocyanate, a cruciferous vegetable-derived cancer chemopreventive agent, is mediated by Bak and Bax, Clin. Cancer Res., 2005, vol. 11, p. 2670. https://doi.org/10.1158/1078-0432.CCR-04-1545
Zhang, Y., Cancer-preventive isothiocyanates: measurement of human exposure and mechanism of action, Mutat. Res., 2004, vol. 555, pp. 173–90. https://doi.org/10.1016/j.mrfmmm.2004.04.017
ACKNOWLEDGMENTS
This work was fully supported by the UGC -RFSMS BSR FELLOWSHIP and DST-PURSE PROGRAMME, Osmania University, Hyderabad, India.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any authors.
Additional information
Publisher’s Note.
Allerton Press remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Sirigiripeta, S., Dokala, A. & Anupalli, R. Synergistic Anti-Cancer Potential of Phenethyl Isothiocyanate and Curcumin Induces Apoptosis and G2/M Cell Cycle Arrest in HER2-Positive Breast Cancer Cells. Cytol. Genet. 57, 611–624 (2023). https://doi.org/10.3103/S0095452723060087
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452723060087