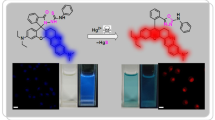
Abstract
Anion-selective detection is demonstrated for sulfate ion in aqueous solutions by using two-photon excited fluorescence of gold nanoparticles (AuNPs) modified with a thiourea-based anion receptor, bis[2-(3-(4-nitrophenyl)thioureido)ethyl]-disulfide. The fluorescent intensity increased with the change of the sulfate concentration in the solution from 10–4 to 10–3 M. In comparison with an unadsorbed receptor molecule in bulk acetonitrile solution, the molecule on AuNPs in water showed improved affinity for sulfate ion. The controllability of the hydrophobicity around receptor molecules on AuNPs is considered a dominant contributing factor for improved sulfate affinity. This unique feature of the surface enables us to detect anionic species in an aqueous phase where a dye-type indicator has poor sensitivity.
Similar content being viewed by others
References
G. M. Pacifici, Early Hum. Dev., 2005, 81, 573.
M. E. Morris and G. Levy, Clin. Pharmacol. Ther., 1983, 33, 529.
H. Shimizu, D. Bolati, A. Adijiang, Y. Adelibieke, G. Muteliefu, A. Enomoto, Y. Higashiyama, Y. Higuchi, F. Nishijima, and T. Niwa, Am. J. Nephrol., 2011, 33, 319.
M. E. Morris and G. Levy, Anal. Biochem., 1988, 172, 16.
B. Buděšinský and L. Krumlová, Anal. Chim. Acta, 1967, 39, 375.
T. Shioya, S. Nishizawa, and N. Teramae, J. Am. Chem. Soc., 1998, 120, 11534.
H. Jeong, E. M. Choi, S. O. Kang, K. C. Nam, and S. Jeon, J. Electroanal. Chem., 2000, 485, 154.
H. R. Seong, D.-S. Kim, S.-G. Kim, H.-J. Choi, and K. H. Ahn, Tetrahedron Lett., 2004, 45, 723.
F.-Y. Wu, Z. Li, L. Guo, X. Wang, M.-H. Lin, Y.-F. Zhao, and Y.-B. Jiang, Org. Biomol. Chem., 2006, 4, 624.
P. Anzenbacher Jr., M. A. Palacios, K. Jursíková, and M. Marquez, Org. Lett., 2005, 7, 5027.
G. K. Darbha, A. K. Singh, U. S. Rai, E. Yu, H. Yu, and P. C. Ray, J. Am. Chem. Soc., 2008, 130, 8038.
R. S. Norman, J. W. Stone, A. Gole, C. J. Murphy, and T. L. Sabo-Attwood, Nano Lett., 2008, 8, 302.
B. Duncan, C. Kim, and V. M. Rotello, J. Controlled Release, 2010, 148, 122.
S. Schlücker, M. Salehi, G. Bergner, M. Schütz, P. Ströbel, A. Marx, I. Petersen, B. Dietzek, and J. Popp, Anal. Chem., 2011, 83, 7081.
W. Eck, G. Craig, A. Sigdel, G. Ritter, L. T. Old, L. Tang, M. F. Brennan, P. J. Allen, and M. D. Mason, ACS Nano, 2008, 2, 2263.
C.-A. Lin, T.-Y. Yang, C. H. Lee, S. H. Huang, R. A. Sperling, M. Zanella, J. K. Li, J.-L. Shen, H.-H. Wang, H.-I. Yeh, W. J. Parak, and W. H. Chang, ACS Nano, 2009, 3, 395.
T. Hayashita, T. Onodera, R. Kato, S. Nishizawa, and N. Teramae, Chem. Commun., 2000, 755.
L. H. Haber, S. J. J. Kwok, M. Semeraro, and K. B. Eisenthal, Chem. Phys. Lett., 2011, 507, 11.
M. Lippitz, M. A. van Dijk, and M. Orrit, Nano Lett., 2005, 5, 799.
G. Ramakrishna, O. Varnavski, J. Kim, D. Lee, and T. Goodson, J. Am. Chem. Soc., 2008, 130, 5032.
K. Boldt, S. Jander, K. Hoppe, and H. Weller, ACS Nano, 2011, 5, 8115.
A. Boulesbaa, A. Issac, D. Stockwell, Z. Huang, J. Huang, J. Guo, and T. Lian, J. Am. Chem. Soc., 2007, 129, 15132.
T. L. Jennings, M. P. Singh, and G. F. Strouse, J. Am. Chem. Soc., 2006, 128, 5462.
K.-Y. Pu, Z. Luo, K. Li, J. Xie, and B. Liu, J. Phys. Chem. C, 2011, 115, 13069.
I. Gryczynski, J. Malicka, Y. Shen, Z. Gryczynski, and J. R. Lakowicz, J. Phys. Chem. B, 2002, 106, 2191.
J. Zhang, T. He, C. Wang, X. Zhang, and Y. Zeng, Opt. Laser Technol., 2011, 43, 974.
S. Yajima, K. Tohda, P. Bühlmann, and Y. Umezawa, Anal. Chem., 1997, 69, 1919.
C. J. Murphy, G. C. Lisensky, L. K. Leung, G. R. Kowach, and A. B. Ellis, J. Am. Chem. Soc., 1990, 112, 8344.
S.-C. Cui, T. Tachikawa, M. Fujitsuka, and T. Majima, J. Phys. Chem. C, 2010, 114, 1217.
J. Tien, A. Terfort, and G. M. Whitesides, Langmuir, 1997, 13, 5349.
X. Ji, X. Song, J. Li, Y. Bai, W. Yang, and X. Peng, J. Am. Chem. Soc., 2007, 129, 13939.
M. R. Beversluis, A. Bouhelier, and L. Novotny, Phys. Rev. B, 2003, 68, 115433.
G. T. Boyd, Z. H. Yu, and Y. R. Shen, Phys. Rev. B, 1986, 33, 7923.
O. P. Varnavski, M. B. Mohamed, M. A. El-Sayed, and T. Goodson, III, J. Phys. Chem. B, 2003, 107, 3101.
K. Imura, T. Nagahara, and H. Okamoto, J. Phys. Chem. B, 2005, 109, 13214.
C. Jiang, Z. Guan, S. Y. R. Lim, L. Polavarapu, and Q.-H. Xu, Nanoscale, 2011, 3, 3316.
A. Kolics and A. Wieckowski, J. Phys. Chem. B, 2001, 105, 2588.
B. Damaskin, A. Frumkin, and A. Chizhov, J. Electroanal. Chem., 1970, 28, 93.
Z. Szklarska-Smialowska and G. Wieczorek, Corros. Sci., 1971, 11, 843.
R. V. Chamberlain II, K. Slowinska, and M. Majda, Langmuir, 2000, 16, 1388.
P. A. Mosier-Boss and S. H. Lieberman, Langmuir, 2003, 19, 6826.
P. A. Mosier-Boss and S. H. Lieberman, Spectrochim. Acta, Part A, 2005, 61, 845.
T. C. Preston, M. Nuruzzaman, N. D. Jones, and S. Mittler, J. Phys. Chem. C, 2009, 113, 14236.
R. Kato, S. Nishizawa, T. Hayashita, and N. Teramae, Tetrahedron Lett., 2001, 42, 5053.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Tomita, K., Ishioka, T. & Harata, A. Development of an Anion Probe: Detection of Sulfate Ion by Two-Photon Fluorescence of Gold Nanoparticles. ANAL. SCI. 28, 1139–1144 (2012). https://doi.org/10.2116/analsci.28.1139
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2116/analsci.28.1139