Abstract
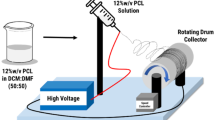
Herein, we fabricated silymarin (SIL)-loaded polycaprolactone (PCL) electrospun nanofiber mats containing different SIL concentrations (5, 7.5, 10%) for wound dressing applications. Solution properties, nanofiber properties and SIL presence were analyzed by viscosity measurements, SEM and FTIR, respectively. Solution viscosities were increased with increasing SIL concentrations resulting in bead-free, thicker and smooth nanofibers. The lowest contact angle was measured as ~ 92° for 10% SIL-loaded sample which had the smoothest nanofibers and a more controlled and continuous SIL release with a rate of 68.29% at end of 144 h during in vitro release experiments. In vivo studies on rats were conducted on this sample and results were compared with a conventional wound dressing and a PCL nanofiber mat. In comparison, the 10% SIL-loaded sample provided more rapid and significantly greater wound healing from the first day of observation. The results confirmed the potential application of PCL/SIL electrospun nanofiber mats as wound dressing.
Graphical abstract








Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and/or from the authors upon reasonable request.
References
A.W. Jatoi, H. Ogasawara, I.S. Kim, Q.Q. Ni, Polyvinyl alcohol nanofiber based three phase wound dressings for sustained wound healing applications. Mater. Lett. 241, 168–171 (2019). https://doi.org/10.1016/j.matlet.2019.01.084
A. Sadeghianmaryan, Z. Yazdanpanah, Y.A. Soltani, H.A. Sardroud, M.H. Nasirtabrizi, X. Chen, Curcumin-loaded electrospun polycaprolactone/montmorillonite nanocomposite: wound dressing application with anti-bacterial and low cell toxicity properties. J. Biomater. Sci. Polym. Ed. 31(2), 169–187 (2020). https://doi.org/10.1080/09205063.2019.1680928
P.D. Kapadnis, S.N. Shrotriya, Electrospun silybin enriched scaffolds of polyethylene oxide as wound dressings: enhanced wound closure, reepithelization in rat excisional wound model. Indian J. Pharm. Educ. Res. 53, 301–309 (2019). https://doi.org/10.5530/ijper.53.2.38
R. Uppal, G.N. Ramaswamy, C. Arnold, R. Goodband, Y. Wang, Hyaluronic acid nanofiber wound dressing-production, characterization, and in vivo behavior. J. Biomed. Mater. Res. B: Appl. Biomater. 97(1), 20–29 (2011). https://doi.org/10.1002/jbm.b.31776
S. Selvaraj, C. Inbasekar, S. Pandurangan, N.F. Nishter, Collagen-coated silk fibroin nanofibers with antioxidants for enhanced wound healing. J. Biomater. Sci. Polym. Ed. 5, 1–18 (2022). https://doi.org/10.1080/09205063.2022.2106707
A. Frenot, I.S. Chronakis, Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 8(1), 64–75 (2003). https://doi.org/10.1016/S1359-0294(03)00004-9
A. Greiner, J.H. Wendorff, Electrospinning: a fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 46(30), 5670–5703 (2007). https://doi.org/10.1002/anie.200604646
M. Abrigo, S.L. McArthur, P. Kingshott, Electrospun nanofibers as dressings for chronic wound care: advances, challenges, and future prospects. Macromol. Biosci. 14, 772–792 (2022). https://doi.org/10.1002/mabi.201300561
A.T. Iacob, M. Drăgan, O.M. Ionescu, L. Profire, A. Ficai, E. Andronescu et al., An overview of biopolymeric electrospun nanofibers based on polysaccharides for wound healing management. Pharmaceutics 12, 983 (2020). https://doi.org/10.3390/pharmaceutics12100983
K. Seethalakshmi, M. Kaviya, B. Venkatachalapathy, S. Mubeena, A.M. Punnoose, T.M. Sridhar, Nanohydroxyapatite-doped polycaprolactone-based nanoscaffolds as a viable drug delivery agent in bone tissue engineering. J. Mater. Res. 36, 420–430 (2021). https://doi.org/10.1557/s43578-020-00042-z
E.G. Choubar, M.H. Nasirtabrizi, F. Salimi, N. Sohrabi-gilani, A. Sadeghianamryan, Fabrication and in vitro characterization of novel co-electrospun polycaprolactone/collagen/polyvinylpyrrolidone nanofibrous scaffolds for bone tissue engineering applications. J. Mater. Res. 37, 4140–4152 (2022). https://doi.org/10.1557/s43578-022-00778-w
M.K. Gaydhane, J.S. Kanuganti, C.S. Sharma, Honey and curcumin loaded multilayered polyvinylalcohol/cellulose acetate electrospun nanofibrous mat for wound healing. J. Mater. Res. 35(600–609), 9 (2020). https://doi.org/10.1557/jmr.2020.52
D.G. Yu, P. Zhao, The key elements for biomolecules to biomaterials and to bioapplications. Biomolecules 12, 1234 (2022). https://doi.org/10.3390/biom12091234
S. Kang, S. Hou, X. Chen, D.G. Yu, L. Wang, X. Li et al., Energy-saving electrospinning with a concentric teflon-core rod spinneret to create medicated nanofibers. Polymers 12(10), 2421 (2020). https://doi.org/10.3390/polym12102421
W. Jiang, X. Zhang, P. Liu, Y. Zhang, W. Song, D.-G. Yu et al., Electrospun healthcare nanofibers from medicinal liquor of Phellinus igniarius. Adv. Compos. Hybrid Mater. 5, 3045–3056 (2022). https://doi.org/10.1007/s42114-022-00551-x
P. Zhao, W. Chen, Z. Feng, Y. Liu, P. Liu, Y. Xie et al., Electrospun nanofibers for periodontal treatment: a recent progress. Int. J. Nanomed. 17, 4137–4162 (2022). https://doi.org/10.2147/IJN.S370340
Y. Zhou, M. Wang, C. Yan, H. Liu, D.G. Yu, Advances in the application of electrospun drug-loaded nanofibers in the treatment of oral ulcers. Biomolecules 12(9), 1254 (2022). https://doi.org/10.3390/biom12091254
F.B. Miguez, O.B.O. Moreira, M.A.L. de Oliveira, Â.M.L. Denadai, L.F.C. de Oliveira, F.B. De Sousa, Reversible electrospun fibers containing spiropyran for acid and base vapor sensing. J. Mater. Res. (2022). https://doi.org/10.1557/s43578-022-00842-5
S.M. Jung, G.H. Yoon, H.C. Lee, H.S. Shin, Chitosan nanoparticle/PCL nanofiber composite for wound dressing and drug delivery. J. Biomater. Sci. Polym. Ed. 26(4), 252–263 (2015). https://doi.org/10.1080/09205063.2014.996699
T.T.T. Nguyen, C. Ghosh, S.G. Hwang, L.D. Tran, J.S. Park, Characteristics of curcumin-loaded poly (lactic acid) nanofibers for wound healing. J. Mater. Sci. 48, 7125–7133 (2013). https://doi.org/10.1007/s10853-013-7527-y
K.K. Chereddy, G. Vandermeulen, V. Préat, PLGA based drug delivery systems: promising carriers for wound healing activity. Wound Repair Regen 24, 223–236 (2016). https://doi.org/10.1111/wrr.12404
H.T. Bui, O.H. Chung, J. Dela Cruz, J.S. Park, Fabrication and characterization of electrospun curcumin-loaded polycaprolactone-polyethylene glycol nanofibers for enhanced wound healing. Macromol. Res. 22, 1288–1296 (2014). https://doi.org/10.1007/s13233-014-2179-6
S.M. Jung, S.K. Min, H.C. Lee, Y.S. Kwon, M.H. Jung, H.S. Shin, Spirulina-PCL nanofiber wound dressing to improve cutaneous wound healing by enhancing antioxidative mechanism. J. Nanomater. (2016). https://doi.org/10.1155/2016/6135727
F. Hejazi, H. Mirzadeh, S. Shojaei, PCL-based 3D nanofibrous structure with well-designed morphology and enhanced specific surface area for tissue engineering application. Prog. Biomater. (2023). https://doi.org/10.1007/s40204-022-00215-5
J.M.A. Mancipe, L.C.B. Pereira, P.G. de Miranda Borchio, M.L. Dias, R.M. da Silva Moreira Thiré, Novel polycaprolactone (PCL)-type I collagen core-shell electrospun nanofibers for wound healing applications. J. Biomed. Mater. Res. Part B: Appl. Biomater. 111, 366–381 (2023). https://doi.org/10.1002/jbm.b.35156
M. Yousefnezhad, S. Davaran, M. Babazadeh, A. Akbarzadeh, H. Pazoki-Toroudi, PCL-based nanoparticles for doxorubicin-ezetimibe co-delivery: a combination therapy for prostate cancer using a drug repurposing strategy. BioImpacts (2023). https://doi.org/10.34172/bi.2023.24252
A. Azari, A. Golchin, M.M. Maymand, F. Mansouri, A. Ardeshirylajimi, Electrospun polycaprolactone nanofibers: current research and applications in biomedical application. Adv. Pharm. Bull. 12(4), 658–672 (2022). https://doi.org/10.34172/apb.2022.070
W. Zeng, N.-m Cheng, X. Liang, H. Hu, F. Luo, J. Jin et al., Electrospun polycaprolactone nanofibrous membranes loaded with baicalin for antibacterial wound dressing. Sci. Rep. 12, 1–11 (2022). https://doi.org/10.1038/s41598-022-13141-0
M.A. Derakhshan, N. Nazeri, K. Khoshnevisan, R. Heshmat, K. Omidfar, Three-layered PCL-collagen nanofibers containing melilotus officinalis extract for diabetic ulcer healing in a rat model. J. Diabetes Metab. Disord. 21, 313–321 (2022). https://doi.org/10.1007/s40200-022-00976-7
R. Ekambaram, S. Saravanan, V.P.S. Babu, S. Dharmalingam, Fabrication and evaluation of Docetaxel doped ZnO nanoparticles incorporated PCL nanofibers for its hemocompatibility, cytotoxicity and apoptotic effects against A549. Materialia 21, 101278 (2022). https://doi.org/10.1016/j.mtla.2021.101278
S. Mozaffari, S. Seyedabadi, E. Alemzadeh, Anticancer efficiency of doxorubicin and berberine-loaded PCL nanofibers in preventing local breast cancer recurrence. J. Drug Deliv. Sci. Technol. 67, 102984 (2022). https://doi.org/10.1016/j.jddst.2021.102984
S. Baghersad, A. Hivechi, S.H. Bahrami, P. Brouki Milan, R.A. Siegel, M. Amoupour, Optimal Aloe vera encapsulated PCL/Gel nanofiber design for skin substitute application and the evaluation of its in vivo implantation. J. Drug Deliv. Sci. Technol. 74, 103536 (2022). https://doi.org/10.1016/j.jddst.2022.103536
S. Eldurini, B.M. Abd El-Hady, M.W. Shafaa, A.A.M. Gad, E. Tolba, A multicompartment vascular implant of electrospun wintergreen oil/polycaprolactone fibers coated with poly(ethylene oxide). Biomed. J. 44, 589–597 (2021). https://doi.org/10.1016/j.bj.2020.04.008
K.P. Devi, Milk thistle (silybum marianum), in Nonvitamin and nonmineral nutritional supplements. (Academic Press, Cambridge, 2019), pp.321–325. https://doi.org/10.1016/B978-0-12-812491-8.00046-1
D. Biedermann, E. Vavříková, L. Cvak, V. Křen, Chemistry of silybin. Nat. Prod. Rep. 31, 1138–1157 (2014). https://doi.org/10.1039/C3NP70122K
A.T. Mpharm, P. Shende, Nanotherapeutic silibinin: an insight of phytomedicine in healthcare reformation. Nanomed. Nanotechnol. Biol. Med. 21, 102057 (2019). https://doi.org/10.1016/j.nano.2019.102057
M. Fallah, A. Davoodvandi, S. Nikmanzar, S. Aghili, S.M.A. Mirazimi, M. Aschner et al., Silymarin (milk thistle extract) as a therapeutic agent in gastrointestinal cancer. Biomed. Pharmacother. 142, 112024 (2021). https://doi.org/10.1016/j.biopha.2021.112024
M. Phiriyawirut, T. Phaechamud, Cellulose acetate electrospun fiber mats for controlled release of silymarin. J. Nanosci. Nanotechnol. 12, 793–799 (2012). https://doi.org/10.1166/jnn.2012.5343
C.S. Chambers, V. Holečková, L. Petrásková, D. Biedermann, K. Valentová, M. Buchta et al., The silymarin composition… and why does it matter??? Food Res. Int. 100, 339–353 (2017). https://doi.org/10.1016/j.foodres.2017.07.017
A. Federico, M. Dallio, C. Loguercio, Silymarin/silybin and chronic liver disease: a marriage of many years. Molecules 22, 191 (2017). https://doi.org/10.3390/molecules22020191
M. Vaid, S. Katiyar, Molecular mechanisms of inhibition of photocarcinogenesis by silymarin, a phytochemical from milk thistle (Silybum marianum L. Gaertn) (Review). Int. J. Oncol. 36(5), 1053–1060 (2010). https://doi.org/10.3892/ijo_00000586
A. Aliabadi, A. Yousefi, A. Mahjoor, M. Farahmand, Evaluation of wound healing activity of silymarin (Silybum marianum) in streptozotocin induced experimental diabetes in rats. J. Anim. Vet. Adv. 10, 3287–3292 (2011). https://doi.org/10.3923/javaa.2011.3287.3292
M. Mahmoodi-Nesheli, S. Alizadeh, H. Solhi, J. Mohseni, M. Mahmoodi-Nesheli, Adjuvant effect of oral Silymarin on patients’ wound healing process caused by thermal injuries. Casp. J. Intern. Med. 9(4), 341–346 (2018). https://doi.org/10.22088/cjim.9.4.341
R. Sharifi, H. Rastegar, M. Kamalinejad, A.R. Dehpour, S.M. Tavangar, M. Paknejad et al., Effect of topical application of Silymarin (Silybum marianum) on excision wound healing in albino rats. Acta Med. Iran. 50(9), 583–588 (2012)
S. Ashkani-Esfahani, Y. Emami, E. Esmaeilzadeh, F. Bagheri, M.R. Namazi, M. Keshtkar et al., Silymarin enhanced fibroblast proliferation and tissue regeneration in full thickness skin wounds in rat models; a stereological study. J. Saudi Soc. Dermatol. Dermatol. Surg. 17, 7–12 (2013). https://doi.org/10.1016/j.jssdds.2012.11.001
A. Oryan, A. Tabatabaei Naeini, A. Moshiri, A. Mohammadalipour, M.R. Tabandeh, Modulation of cutaneous wound healing by silymarin in rats. J. Wound Care 21(9), 457–464 (2012). https://doi.org/10.12968/jowc.2012.21.9.457
V. Jacobs, R.D. Anandjiwala, M. Maaza, The influence of electrospinning parameters on the structural morphology and diameter of electrospun nanofibers. J. Appl. Polym. Sci. 115, 3130–3136 (2010). https://doi.org/10.1002/app.31396
V. Beachley, X. Wen, Effect of electrospinning parameters on the nanofiber diameter and length. Mater. Sci. Eng. C 29, 663–668 (2009). https://doi.org/10.1016/j.msec.2008.10.037
Z. Li, C. Wang, Effects of working parameters on electrospinning, in One-dimensional nanostructures. ed. by Z. Li, C. Wang (Berlin, Springer, 2013), pp.15–28. https://doi.org/10.1007/978-3-642-36427-3_2
Y. Yuan, T.R. Lee, Contact angle and wetting properties, in Surface science techniques, vol. 51, ed. by G. Bracco, B. Holst (Berlin, Springer, 2013), pp.3–34
T. Darmanin, F. Guittard, Wettability of conducting polymers: from superhydrophilicity to superoleophobicity. Prog. Polym. Sci. Pergamon. 39, 656–682 (2014). https://doi.org/10.1016/j.progpolymsci.2013.10.003
M.M. Shaik, A. Dapkekar, J.M. Rajwade, S.H. Jadhav, M. Kowshik, Antioxidant-antibacterial containing bi-layer scaffolds as potential candidates for management of oxidative stress and infections in wound healing. J. Mater. Sci. Mater. Med. 30, 1–13 (2019). https://doi.org/10.1007/s10856-018-6212-8
A.E. El-Nahas, A.N. Allam, D.A. Abdelmonsif, A.H. El-Kamel, Silymarin-loaded Eudragit nanoparticles: formulation, characterization, and hepatoprotective and toxicity evaluation. AAPS PharmSciTech 18, 3076–3086 (2017). https://doi.org/10.1208/s12249-017-0799-9
T. Elzein, M. Nasser-Eddine, C. Delaite, S. Bistac, P. Dumas, FTIR study of polycaprolactone chain organization at interfaces. J. Colloid. Interface Sci. 273, 381–387 (2004). https://doi.org/10.1016/j.jcis.2004.02.001
T. Khampieng, G.E. Wnek, P. Supaphol, Electrospun DOXY-h loaded-poly(acrylic acid) nanofiber mats: in vitro drug release and antibacterial properties investigation. J. Biomater. Sci. Polym. Ed. 25, 1292–1305 (2014). https://doi.org/10.1080/09205063.2014.929431
M. Padmaa Paarakh, P. Ani Jose, C.M. Setty, G.V.P. Christoper, Release kinetics-concepts and applications. Int. J. Pharm. Res. Technol. 8(1), 12–20 (2018). https://doi.org/10.31838/ijprt/08.01.02
P.F. Surai, Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants 4(1), 204–247 (2015). https://doi.org/10.3390/antiox4010204
E.S. Lovelace, J. Wagoner, J. MacDonald, T. Bammler, J. Bruckner, J. Brownell et al., Silymarin suppresses cellular inflammation by inducing reparative stress signaling. J. Nat. Prod. 78, 1990–2000 (2015). https://doi.org/10.1021/acs.jnatprod.5b00288
J. Zhao, M. Lahiri-Chatterjee, Y. Sharma, R. Agarwal, Inhibitory effect of a flavonoid antioxidant silymarin on benzoyl peroxide-induced tumor promotion, oxidative stress and inflammatory responses in SENCAR mouse skin. Carcinogenesis 21, 811–816 (2000). https://doi.org/10.1093/carcin/21.4.811
G. Zhu, D. Kremenakova, Y. Wang, J. Militky, Air permeability of polyester nonwoven fabrics. Autex Res. J. 15, 8–12 (2015). https://doi.org/10.2478/aut-2014-0019
Z. Ma, M. Kotaki, T. Yong, W. He, S. Ramakrishna, Surface engineering of electrospun polyethylene terephthalate (PET) nanofibers towards development of a new material for blood vessel engineering. Biomaterials 26, 2527–2536 (2005). https://doi.org/10.1016/j.biomaterials.2004.07.026
P. Costa, J.M. Sousa Lobo, Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 13, 123–133 (2001). https://doi.org/10.1016/S0928-0987(01)00095-1
C. Aras, E.T. Ozer, G. Goktalay, G. Saat, E. Karaca, Evaluation of Nigella sativa oil loaded electrospun polyurethane nanofibrous mat as wound dressing. J. Biomater. Sci. Polym. Ed. 32(13), 1718–1735 (2021). https://doi.org/10.1080/09205063.2021.1937463
Acknowledgments
Authors would like to thank Erkan Ermis for his technical assistance during the in vivo studies and Sema Isik for her technical support during the in vitro studies. This study is a part of Aisegkioul Sali’s MSc thesis and was financially supported by The Scientific Research Commission of Bursa Uludag University with the project number of THIZ-2021-680.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sali, A., Duzyer Gebizli, S. & Goktalay, G. Silymarin-loaded electrospun polycaprolactone nanofibers as wound dressing. Journal of Materials Research 38, 2251–2263 (2023). https://doi.org/10.1557/s43578-023-00959-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-023-00959-1