Abstract
Background
Neoadjuvant chemoimmunotherapy treatment (NCIT) has achieved great success for non-small cell lung cancer (NSCLC); however, the intrinsic mechanism underlying this treatment remains unclear.
Methods
Thirty-two patients with stage IIA–IIIC NSCLC who underwent surgery after NCIT were included in this retrospective study. Multiplex immunofluorescence (mIF) staining and image analysis assays were performed on the samples collected before and after NCIT for each patient. RNA analyses was applied to confirm the mIF results.
Results
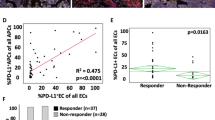
Among the enrolled patients, 14 achieved major pathological response or pathological complete response (pCR) and were defined as the ‘response’ group, whereas 18 patients did not respond well to NCIT and were defined as the ‘nonresponse’ group. The results of the mIF assays revealed an overall increase in tumor immune lymphocytes (TILs) after NCIT in the stroma area (p = 0.03) rather than the tumor area (p = 0.86). The percentage of CD8+ T cells and tertiary lymphoid structure counts in both the response and nonresponse groups increased significantly after NCIT compared with before NCIT. CD3+ T cells and FOXP3+ cells decreased significantly in the response group but remained unchanged or increased in the nonresponse group. A comparison of the response and nonresponse groups showed that CD3, FOXP3+ and CD8+/PD-1+ cells before NCIT may serve as predictors of the response to neoadjuvant immunotherapy. The RNA analyses confirmed the mIF results that TILs were elevated after NCIT.
Conclusions
The infiltration of immune cells before NCIT was correlated with pathologic complete response, which enhanced the TILs as a promising predictor for selecting patients who were more likely to benefit from NCIT.






Similar content being viewed by others
Data Availability
Sequence Archive for National Genomics Data Center (BioProject ID: PRJCA013526, https://ngdc.cncb.ac.cn/bioproject/browse/PRJCA013526; OMIX ID: OMIX002464, https://ngdc.cncb.ac.cn/omix/release/OMIX002464). The data generated and analyzed during this study are described in the ESM (Tables S1 and S2).
References
Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. https://doi.org/10.1016/S0140-6736(18)32409-7.
Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. https://doi.org/10.1056/NEJMoa1801005.
Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65. https://doi.org/10.1016/S0140-6736(16)32517-X.
Kim S, Buecher B, Andre T, et al. Atezolizumab plus modified docetaxel-cisplatin-5-fluorouracil (mDCF) regimen versus mDCF in patients with metastatic or unresectable locally advanced recurrent anal squamous cell carcinoma: a randomized, non-comparative phase II SCARCE GERCOR trial. BMC Cancer. 2020;20(1):352. https://doi.org/10.1186/s12885-020-06841-1.
Wang Z, Duan J, Cai S, et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol. 2019;5(5):696–702. https://doi.org/10.1001/jamaoncol.2018.7098.
Wang Z, Zhao X, Gao C, et al. Plasma-based microsatellite instability detection strategy to guide immune checkpoint blockade treatment. J Immunother Cancer. 2020;8(2):e001297. https://doi.org/10.1136/jitc-2020-001297.
Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage iv or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–26. https://doi.org/10.1056/NEJMoa1613493.
Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24(9):1441–8. https://doi.org/10.1038/s41591-018-0134-3.
Forde PM, Spicer J, Lu S, et al. Abstract CT003: nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo as neoadjuvant treatment (tx) for resectable (IB-IIIA) non-small cell lung cancer (NSCLC) in the phase 3 CHECKMATE 816 trial. Cancer Res. 2021;81:C003.
Liu Z, Gao Z, Zhang M, et al. Real-world effectiveness and prognostic factors analysis of stages I–III non-small cell lung cancer following neoadjuvant chemo-immunotherapy or neoadjuvant chemotherapy. Ann Thorac Cardiovasc Surg. 2022;28(2):111–20. https://doi.org/10.5761/atcs.oa.21-00143.
Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):786–95. https://doi.org/10.1016/S1470-2045(20)30140-6.
Fumet JD, Richard C, Ledys F, et al. Prognostic and predictive role of CD8 and PD-L1 determination in lung tumor tissue of patients under anti-PD-1 therapy. Br J Cancer. 2018;119(8):950–60. https://doi.org/10.1038/s41416-018-0220-9.
Kim SR, Chun SH, Kim JR, et al. The implications of clinical risk factors, CAR index, and compositional changes of immune cells on hyperprogressive disease in non-small cell lung cancer patients receiving immunotherapy. BMC Cancer. 2021;21(1):19. https://doi.org/10.1186/s12885-020-07727-y.
Hwang I, Kim JW, Ylaya K, et al. Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients. J Transl Med. 2020;18(1):443. https://doi.org/10.1186/s12967-020-02618-z.
Liu Y, Zugazagoitia J, Ahmed FS, et al. Immune cell PD-L1 colocalizes with macrophages and is associated with outcome in PD-1 pathway blockade therapy. Clin Cancer Res. 2020;26(4):970–7. https://doi.org/10.1158/1078-0432.CCR-19-1040.
Gaudreau PO, Negrao MV, Mitchell KG, et al. Neoadjuvant chemotherapy increases cytotoxic T cell, tissue resident memory T cell, and B cell infiltration in resectable NSCLC. J Thorac Oncol. 2021;16(1):127–39. https://doi.org/10.1016/j.jtho.2020.09.027.
Cascone T, William WN Jr, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med. 2021;27(3):504–14. https://doi.org/10.1038/s41591-020-01224-2.
Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. https://doi.org/10.1016/j.jtho.2015.09.009.
Travis WD, Dacic S, Wistuba I, et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol. 2020;15(5):709–40. https://doi.org/10.1016/j.jtho.2020.01.005.
Dai Y, Zhao L, Hua D, et al. Tumor immune microenvironment in endometrial cancer of different molecular subtypes: evidence from a retrospective observational study. Front Immunol. 2022;13:1035616. https://doi.org/10.3389/fimmu.2022.1035616.
Ju WT, Xia RH, Zhu DW, et al. A pilot study of neoadjuvant combination of anti-PD-1 camrelizumab and VEGFR2 inhibitor apatinib for locally advanced resectable oral squamous cell carcinoma. Nat Commun. 2022;13(1):5378. https://doi.org/10.1038/s41467-022-33080-8.
Zhang W, Gong C, Peng X, et al. Serum concentration of CD137 and tumor infiltration by M1 macrophages predict the response to Sintilimab plus bevacizumab biosimilar in advanced hepatocellular carcinoma patients. Clin Cancer Res. 2022;28(16):3499–508. https://doi.org/10.1158/1078-0432.CCR-21-3972.
Yu J, Yan J, Guo Q, et al. Genetic aberrations in the CDK4 pathway are associated with innate resistance to PD-1 blockade in Chinese patients with non-cutaneous melanoma. Clin Cancer Res. 2019;25(21):6511–23. https://doi.org/10.1158/1078-0432.ccr-19-0475.
Koeppen H, Yu W, Zha J, et al. Biomarker analyses from a placebo-controlled phase II study evaluating erlotinib+/−onartuzumab in advanced non-small cell lung cancer: MET expression levels are predictive of patient benefit. Clin Cancer Res. 2014;20(17):4488–98. https://doi.org/10.1158/1078-0432.CCR-13-1836.
Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577(7791):549–55. https://doi.org/10.1038/s41586-019-1922-8.
Sumitomo R, Hirai T, Fujita M, Murakami H, Otake Y, Huang CL. M2 tumor-associated macrophages promote tumor progression in non-small-cell lung cancer. Exp Ther Med. 2019;18(6):4490–8. https://doi.org/10.3892/etm.2019.8068.
Qin H, Wang F, Liu H, et al. New advances in immunotherapy for non-small cell lung cancer. Am J Transl Res. 2018;10(8):2234–45.
Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol. 2012;33(6):297–305. https://doi.org/10.1016/j.it.2012.04.006.
Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014;14(7):447–62. https://doi.org/10.1038/nri3700.
Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19(6):307–25. https://doi.org/10.1038/s41568-019-0144-6.
Dieu-Nosjean MC, Goc J, Giraldo NA, Sautes-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014;35(11):571–80. https://doi.org/10.1016/j.it.2014.09.006.
Cabrita R, Lauss M, Sanna A, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577(7791):561–5. https://doi.org/10.1038/s41586-019-1914-8.
Petitprez F, de Reynies A, Keung EZ, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577(7791):556–60. https://doi.org/10.1038/s41586-019-1906-8.
Vanhersecke L, Brunet M, Guegan JP, et al. Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression. Nat Cancer. 2021;2(8):794–802. https://doi.org/10.1038/s43018-021-00232-6.
Thaunat O, Field AC, Dai J, et al. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci USA. 2005;102(41):14723–8. https://doi.org/10.1073/pnas.0507223102.
Thaunat O, Patey N, Caligiuri G, et al. Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J Immunol. 2010;185(1):717–28. https://doi.org/10.4049/jimmunol.0903589.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (82072564, 82272679), Project of Shanghai Natural Science Foundation (20ZR1452000), Program of Shanghai Academic Research Leader (22XD142280), Shanghai Municipal Health Commission (2022XD029), Shanghai Youth Talent Support Program, Shanghai Talent Development Fund (2019073), Shanghai Chest Hospital Project of Collaborative Innovative Grant (YJT20190209), The Innovative Research Team of High-level Local Universities in Shanghai (SHSMU-ZLCX20212302), Lian Yun Gang Shi Hui Lan Public Foundation (HL-HS2020-65), Guangdong Association of Clinical Trials (GACT)/Chinese Thoracic Oncology Group (CTONG) and Guangdong Provincial Key Lab of Translational Medicine in Lung Cancer (2017B030314120), National Multi-disciplinary Treatment Project for Major Diseases (2020NMDTP), and Shanghai Chest Hospital Project of Talent Support (2018YNZYJ02)
Author information
Authors and Affiliations
Contributions
TC and ZC contributed equally to this work. YS, JH, SS, SL and ZL participated in the design and performance of this study. FW, XZ and DZ participated in the analysis and interpretation of the data. YC, HC and MH performed the statistical analysis. The manuscript was drafted by TC, FW and DZ, and was reviewed by all authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Disclosures
Fengcai Wen, Xiaochen Zhao, Ding Zhang, Mengli Huang, Yanan Chen, and Hao Chen were employed by 3D Medicines-Shanghai Inc. Tianxiang Chen, Zhengqi Cao, Yingjia Sun, Jia Huang, Shengping Shen, Yueping Jin, Long Jiang, Shun Lu, and Ziming Li declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, T., Cao, Z., Sun, Y. et al. Neoadjuvant Chemoimmunotherapy Increases Tumor Immune Lymphocytes Infiltration in Resectable Non-small Cell Lung Cancer. Ann Surg Oncol 30, 7549–7560 (2023). https://doi.org/10.1245/s10434-023-14123-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14123-w