Abstract
Background
Modified docetaxel, cisplatin, and 5-fluorouracil (mDCF) regimen has become a new standard for the treatment of metastatic or unresectable locally advanced recurrent squamous cell carcinoma of the anus (SCCA) after demonstrating improved efficacy (12-month PFS of 47%) in the Epitopes-HPV02 trial. Antibodies targeting the checkpoint inhibitor (CKI) programmed cell death protein-1 (PD1) have demonstrated the efficacy as monotherapies in second-line treatment of SCCA. The aim of this study is to evaluate the combination of atezolizumab and mDCF as first-line chemotherapy in a non-comparative multicentre randomized phase II study of advanced SCCA patients.
Methods
Patients with chemo-naive advanced histologically proven SCCA, metastatic or unresectable locally advanced recurrence, and Eastern Cooperative Oncology Group-performance status (ECOG-PS) < 2 will be eligible. The primary endpoint is a 12-month PFS rate. Using one-arm non-parametric survival with unilateral alpha type I error of 5% and a statistical power of 80%, the upper critical value for the 12-month PFS rate is 47% to reject H0. Assuming 5% lost to follow-up, 99 patients will be randomized on a 2:1 basis, 66 to the experimental arm (arm A, mDCF plus atezolizumab) and 33 to the standard arm (arm B, mDCF). In both arms, 8 cycles of mDCF will be administered. In arm A, patients receive mDCF with a fixed dose of atezolizumab (800 mg every 2 weeks) and are followed up to 1 year. Secondary endpoints are overall survival, PFS, response rate, safety, health-related quality of life, and an extensive biomarker programme and its correlation with the treatment efficacy.
Discussion
Although the Epitopes-HPV02 trial has changed long-lasting prognosis of patients with SCCA in advanced stage disease, more than 50% of patients will progress at 12 months. The purpose of the SCARCE trial to establish the addition of atezolizumab to mDCF as a new standard in this rare disease. Associated biomarker studies and the control arm could contribute to better understanding of the potential synergic and tumour resistance mechanisms in SCCA.
Trial registration
Similar content being viewed by others
Background
Squamous cell carcinoma of the anus (SCCA) is still considered as a rare disease accounting for less than 3% of all gastrointestinal tumours [1], but the annual incidence rate is increasing worldwide mostly due to its association with human papillomavirus (HPV) infection, predominantly with the genotype HPV-16 [2]. Approximately 15% of patients are diagnosed at an advanced stage [3] and 25–40% will experience disease progression after curative intent chemoradiotherapy (CRT) for localized disease [4]. In patients with non-resectable local recurrences or with distant metastases, the systemic chemotherapy is the standard approach.
Recently, the modified docetaxel, cisplatin, and 5-fluorouracil (mDCF) combination chemotherapy regimen has demonstrated high efficacy, with good tolerance in a phase II Epitopes-HPV02 trial of 69 SCCA patients [5] and thus has become the first validated chemotherapy regimen in advanced SCCA. The objective response rate (ORR) was as high as 90.9%, with complete response of 45.5%. The median PFS was 11 months and the median overall survival (OS) was still not reached at a median follow-up of 33.4 months [6]. In addition, mDCF demonstrated significantly lower toxicity compared to the standard DCF (Grade III/IV toxicity rate of 53% vs 83%) while maintaining the same efficacy [5]. More recently, these encouraging results were externally validated with the updated data from the Epitopes-HPV02 trial including 51 SCCA patients. The ORR reached 85.1% (40 of 47 evaluable patients), with a complete response of 34% (16 of 47 patients). The median PFS was 12.7 months (95% CI, 11.2–34.5) and the median OS was 50.2 months (95% CI, 21.4–120.0) [6]. In addition, the translational research program demonstrated the ability of DCF to induce clearance of HPV oncoprotein-related DNA in patient blood (HPV circulating tumour DNA [ctDNA]), which was correlated with long-term remissions. In SCCA, 95% of cases are related to HPV, mostly to genotype HPV16 (89%) [7]. HPV-negative SCCA tumors are rare, frequently TP53-mutated, and have a worse prognosis [8, 9]. In HPV-related cancers, HPV viral genomes are usually integrated into the tumour cell genome or episomal DNA. Droplet-digital PCR (ddPCR) detects and quantifies HPV ctDNA at baseline in almost all advanced stage patients, and in 0% of healthy controls as well as women treated for HPV16-associated high grade intraepithelial neoplasia [10]. In the Epitopes-HPV02 trial, HPV ctDNA was detected at baseline in 91% of patients. After treatment with DCF, residual HPV ctDNA was present in 39% of patients and was highly predictive of disease progression (HR = 5.5; p < 0.001) and OS (HR = 7.0; p = 0.02) [11], validating the interest of HPV ctDNA in routine clinical practice in advanced SCCA patients [12].
Another biomarker of interest in this disease setting is telomerase reverse transcriptase (TERT). TERT is an active enzyme during embryonic foetal development, but not in non-self-renewing somatic cells. Our previously data supports the use of TERT as an HPV-related antigen in SCCA [5]. We observed that TERT-specific immune responses significantly influence OS in SCCA patients after DCF chemotherapy (p = 0.040) in comparison with HPV-specific immune response (p = 0.09) [5].
Besides, the carboplatin and paclitaxel (CP) combination regimen has demonstrated an improved safety compared to cisplatin and 5-fluorouracil (5-FU; CF) in the InterAACT randomized phase II trial of 90 SCCA patients. Although grade III/IV adverse events rate was similar (71–74%) between both arms, there were significantly less severe adverse events with CP (36%) compared to CF (62%). However, this trial has failed to meet its primary endpoint; ORR was 59 and 57.1% with CP and CF, respectively [13].
Despite these practice-changing results, 53% of patient treated with DCF and approximately 85% of patients treated with CP will present disease-progression at 12 months; and at date, there is no validated chemotherapy regimen in second-line. The programmed cell death protein-1 immune checkpoint (PD1) and its ligand (PD-L1) are particularly relevant target candidate for immunotherapy in SCCA patients, based on the prominent role of PD-1/PD-L1 in HPV-driven immune-evasion [14,15,16]. Two prospective non-controlled trials have shown the interest of immunotherapies in this disease [17, 18]. Nivolumab and pembrolizumab, two antibodies targeting PD1, had demonstrated antitumour activity in refractory patients to at least one line of chemotherapy. Objective responses were observed in 20% of patients, with estimated 12-month PFS and OS rates of 20 and 48%, respectively [17, 18].
The association of an anti-PD1/PD-L1 with chemotherapy seems promising. The combination of different chemotherapy regimens and an anti-PD1/PD-L1 were feasible with improved survival in first-line advanced small-cell and non-small-cell lung cancers [19,20,21]. In anal carcinoma, the Epitopes-HPV02 trial showed that the mDCF regimen was feasible with 53% of grade 3–4 adverse-events, with no grade 4 non-haematological toxicities, and without febrile neutropenia. Given that mDCF may enhance antitumour immune response, it was recommended as an interesting candidate to be evaluated as a backbone chemotherapy for immunotherapy combinations in SCCA [5].
Therefore, we decided to associate a PD1/PD-L1 inhibitor to the mDCF-based chemotherapy regimen to improve the efficacy with higher rate of long-lasting progression-free survivors. The associated extensive ancillary biomarker studies of tumour tissues and peripheral blood samples will provide a unique opportunity to assess a potential synergy mechanism between mDCF and checkpoint inhibitor (CKI) and will help to improve our knowledge about underlying resistance mechanisms.
Methods and analysis
SCARCE is a randomized, non-comparative, multicentre phase II trial to evaluate the combination of atezolizumab and the mDCF regimen in advanced SCCA patients. The study was developed by the “National Institute of Health and Medical Research (INSERM), Unit 1098”, and “Clinical Investigational Center (CIC) 1431”. It is coordinated and sponsored by the GERCOR Medical Oncology group and supported by the PRODIGE collaborative oncologic group. The data management is undertaken by the “Methodology and Quality of Life Unit in Oncology”* of the University Hospital of Besançon. The trial is registered on the clinicaltrials.gov (NCT03519295) and is conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice (GCP).
* http://www.umqvc.org/en/index.html
Study objectives
The primary endpoint is to evaluate the observed PFS rate at 12 months from the initiation of DCF in patients with metastatic or unresectable locally advanced recurrent SCCA. PFS is defined as the time from randomization to progression (evaluated by the RECIST criteria version 1.1) or death from any cause, whichever occurred first.
The secondary endpoints are:
To evaluate OS,
To evaluate PFS,
To evaluate health-related quality of life (HRQoL),
To evaluate ORR,
To evaluate the tolerance of DCF in in association with atezolizumab,
To evaluate the predictive value of HPV-specific and telomerase-specific T cell responses monitored before and after treatment,
To analyse HPV, p53, and neo-antigens genotypes and their correlation with the treatment efficacy,
To investigate the impact of peripheral immune system status (Treg, CD4+ polarization, myeloid-derived suppressor cells [MDSC], T-cell exhaustion) on clinical outcomes and HPV/telomerase specific immunity,
To investigate the prognostic value of tumour-infiltrating lymphocytes and PD-L1 expression,
To explore the correlation of both peripheral CD4+ anti-telomerase immunity and PDL1 immunohistochemistry with PFS,
To characterize the predictive value of soluble biomarkers (e.g. soluble PD-L1) and plasmatic HPV DNA monitoring,
To evaluate the correlation between neo-antigen burden and survival at 12 months.
Patient selection
The study population consists of patients with histologically proven SCCA at advanced stage defined as:
Stage IV disease with distant metastases, or
Locally advanced recurrence after CRT, non-eligible for salvage surgery due to the extension of the disease.
Patients should have an Eastern Cooperative Oncology Group (ECOG) Performance ...
(ECOG-PS) of 0 or 1 and adequate organ functions. The inclusion and exclusion criteria are listed in Table 1.
Treatments
Atezolizumab
Patients in the experimental arm (arm A) receive atezolizumab every 2 weeks for a total of 24 cycles at a fixed dose of 800 mg as 60 min IV infusion before mDCF, for 8 first cycles followed by atezolizumab monotherapy. If treatment is well tolerated at first cycle, atezolizumab perfusion can be reduced to 30 min in the following cycles. No dose adjustment is required. No premedication treatment is required before infusion of atezolizumab.
mDCF
Patients in both arms receive 8 cycles of mDCF (docetaxel 40 mg/m2 day 1, cisplatin 40 mg/m2 day 1 and 5-FU at 1200 mg/m2/over 2 days) every 2 weeks.
Given the low-risk (< 10%) of febrile neutropenia with the mDCF protocol, support of granulocyte colony-stimulating factor (G-CSF) for 4 days after mDCF administration is recommended (day 4- day 7) only as secondary prophylaxis.
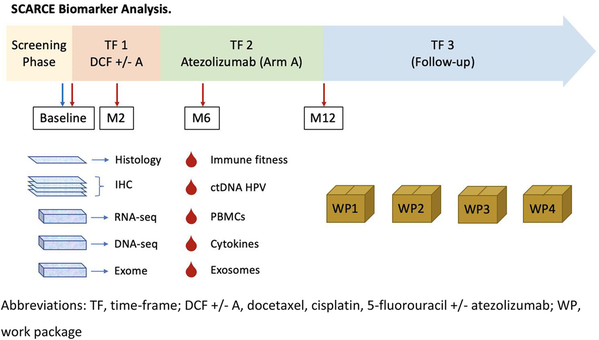
Study description (Fig. 1)
Therapeutic seqssssuence
- Initial assessments will be performed at baseline, within 28 days prior to the first administration of treatment.
Schedule of enrolment, interventions, and assessments. a Clinical examination: height (at baseline only), weight, and ECOG-PS. b Vital signs: pulse, blood pressure and body temperature. c Blood analysis: complete blood count including red blood cells, haemoglobin, haematocrit, lymphocytes, white blood cells and differentials and platelets, blood electrolytes, bicarbonates, glycemia, proteinemia, albumin, blood urea nitrogen, creatinine, creatinine clearance (MDRD), calcium, magnesium, AST, ALT, gamma-GT, conjugated and total bilirubin, ALP, TSH, LDH, and C-reactive protein. d PET-scan at baseline, at first visit of phase 2 and at the end-point visit (except in case of early progression). CT-scans and PET-scans will be collected for a central review. e The EORTC-QLQC30 questionnaire: every second chemotherapy cycles in phase 1, and every 2 months during phase 2 until the end-point visit. f Blood samples for biomonitoring: 1 EDTA tube of 6 ml (plasma) and 8 EDTA tubes of 6 ml (PBMC). g According to the NCI-CTCAE guidelines version 4.03. h Only in HIV-positive patients. £ Immunotherapy alone: to be continued 1 cycle every to weeks up to a maximum of 12 months from the randomization date. * The end-point visit: to be performed 12 months after randomization or 4 weeks after the last chemotherapy cycle in case of premature end of treatment (for toxicity, progression, or patient or physician decision). $ The follow-up visit: to be performed every 3 months from the end-point visit to a patient death or at least 3 years after randomization
- Patients will be randomized via the electronic case report form (eCRF) and allocated to a treatment on a 2:1 basis into one of the two arms:
Arm A (66 patients): 8 cycles of the mDCF regimen every 2 weeks in association with atezolizumab administrated every 2 weeks for 12 months.
Time-frame (TF) 1: 8 cycles of mDCF + atezolizumab (4 months).
TF 2: 16 cycles of atezolizumab alone (8 months).
TF 3: follow-up every 3 months for 2 years
Arm B (33 patients): 8 cycles of the mDCF regimen every 2 weeks.
TF 1: 8 cycles of mDCF (4 months).
TF 2: follow-up every 2 months (8 months).
TF 3: follow-up every 3 months for 2 years.
Evaluation, laboratory tests, and follow-up
-
Computed tomography (CT-scan) will be planned at baseline and every 8 weeks until 12 months (or disease progression) from randomization (TF 1 and 2) and every 12 weeks thereafter (TF 3) in both arms,
-
Positron emission tomography (PET) scan will be performed at baseline, at the end of mDCF treatment, and at 12 months after randomization (in absence of disease progression),
-
Surgery and/or palliative radiotherapy of residual metastatic sites are allowed according to the Investigator’s centre practices after week 20,
-
The end of treatment visit will be performed 12 months after randomization or 4 weeks after the last cycle of treatment if treatment is stopped prematurely (progression, toxicity, or decision of physician or patient),
-
Follow-up every 3 months after the end of treatment visit until patient death or at least 3 years after the randomization date.
Randomization
Once the patient’s consent is obtained, the Investigator will confirm that all required radiological and biological procedures were performed within schedule before randomization.
Patients will be randomized in a 2:1 ratio to receive mDCF and atezolizumab (Arm A) or mDCF (Arm B).
The randomization will be open following the minimization technic (hazard compound: 0.8) with stratification factors as follows:
Age (65 < years vs ≥ 65 years),
Synchronous (metastases at the time of diagnosis) vs metachronous (metastases after starting a treatment for a localized disease) vs locally advanced unresectable disease and no evidence of metastases.
The attribution of the treatment arm will be performed centrally.
Randomization will be performed based on an eCRF form.
Quality of life assessment
Health-related quality of life (HRQoL) EORTC QLQ-C30 questionnaire will be collected:
At inclusion, before randomization,
Every 2 cycles of chemotherapy in TF 1 (mDCF),
Every 2 months in TF 2 until the end of treatment visit,
At the end of treatment visit,
Every 3 months during follow-up in TF 3, until patient death, or at least 3 years after the randomization date.
The EORTC QLQ-C30 questionnaire is validated in French [22]. It consists of 30 items measuring five functional scales (physical, role, emotional, cognitive, and social functioning), a global health status, financial difficulties, and eight scales of symptoms (fatigue, nausea and vomiting, pain, dyspnoea, insomnia, appetite loss, constipation, and diarrhoea). One score is generated per dimension and standardized on a 0 to 100 scale in order that a high score reflects a high GHS, functional and symptomatic level.
Biological sample collection
Four blood samples will be collected at:
Sample N°1: At inclusion visit before the first treatment.
Sample N°2: At 2 months from randomization.
Sample N°3: At 6 months after randomization.
Sample N°4: At the end-point visit.
Biomonitoring Blood Samples: seven 6 ml ethylenediamine tetra-acetic acid (EDTA) tubes and two 4 ml EDTA tubes will be collected for analysis.
Tumour samples
Tumour samples obtained by biopsies or surgery at diagnosis will be centralized for translational research program.
Data management
For each patient enrolled in the study, the Investigators must document all required data in the corresponding source documents. These data must then be entered into eCRF, which will be accessible only by authorized persons via secured web connection. One eCRF will be created for each patient. The investigator has the responsibility for its completion, proof reading, as well as its approval after the final verification for the authenticity and accuracy of all entered data. The Monitor, who is mandated by the Sponsor, will ensure that the study is conducted in accordance with the GCP guidelines and all applicable local laws and that the rights, security, and well-being of the patient are respected. The Monitor will perform source document verification and validation and request clarification to ensure the accuracy, completeness, and reliability of data. The Investigator guarantees the Sponsor or its representative direct access to source documents. Throughout the study, data electronically captured via eCRF will be regularly checked for consistency, and queries on data clarification will be generated through eCRF. At the end of the data handling process, a data review meeting will be held to prepare the database lock. After database lock, data will be transferred into SAS format to produce statistical analyses.
Statistical considerations
Planed number of patients to include
This study is a 2:1 randomized non-comparative multicentre two-arm phase II study.
The Arm B will serve as calibration arm that the populations in the two arms are similar and to validate the H0 hypothesis in Arm A: no statistical comparison is planned between the two arms. Results acquired in Arm B will also allow the development of a translational investigation to identify potential biomarker for atezolizumab and the mDCF combination in HPV-related diseases and to generate reliable hypothesis for biomarker study.
Then for experimental arm (arm A) we consider the following hypotheses:
H0: a PFS rate at 12 months of 35% is uninteresting,
H1: a PFS rate at 12 months of 50% is expected.
Using one arm non-parametric survival with unilateral alpha type I error of 5 and 80% statistical power 62 patients will be required to in 2 years with a 12-month follow-up. The Upper Critical Value for the 12-month PFS rate is 47% to reject H0.
Given 5% lost to follow-up, 66 patients should be randomized in the experimental arm (Arm A) and according to a ratio 2:1, 33 patients into the standard arm (Arm B), for a total of 99 patients.
Modality of analysis
The primary analysis will be made on modified intention-to-treat (mITT) population, i.e. including all evaluable randomized patients regardless of their eligibility and treatment received. The results will be reported according to the randomized treatment.
A final statistical plan and a specific statistical plan dedicated to HRQoL analyses will be written before data frozen.
The mITT population will be used for the analyses of all efficacy endpoints. mITT2 population will be used for HRQoL analyses.
The qualitative variables for each modality will be described using numbers and percentages (in relation to the effective). The quantitative variables will be described using the medians, averages, standard deviations, extreme values, and interquartile. For each variable, the number of missing data will be specified. The confidence intervals at 95% (95% CI) will be reported.
The 12-month rate and the median PFS with their 90% CI will be estimated using the Kaplan-Meier method (73). The PFS rates at specified time points will be estimated from the Kaplan-Meier curve and the standard errors by the Greenwood formula. The log-log transformation will be used to compute CIs.
OS and its 95% CI will be calculated between the date of randomization and the date of death from any cause. It will be estimated according to by the Kaplan Meier method. Alive patients or lost to follow-up at the time of the analysis will be censored at the date of last follow-up.
In an exploratory analysis, a hazard ratio (HR) will be estimated using a univariate Cox model with its 90% CI. Independent factors associated with OS (types of metastases, response to the treatment, HRQoL, etc.) will be explored using univariate and multivariate Cox models.
All analyses of HRQoL data will be done on mITT2 population (population mITT1 with at least on HRQoL data available).
HRQoL scores will be described according to the treatment arms with the means (standard deviation) and medians (range Min-Max).
Study schedule (Fig. 1)
Baseline
To be performed within 28 days prior to the initiation of treatment:
Inclusion/exclusion criteria check, informed consent collection, and HIV serology,
Clinically relevant medical/surgical history and associated therapies data collection,
CT-scan and positron emission tomography (PET)-scan (if PET-scan performed more than 28 days prior to the initiation of treatment, it could be validated by the Coordinator considering the accessibility to PET-scan in given centre),
Tumour sample collection: tumour sample realized in routine will be collected,
To be performed within 7 days prior to the initiation of treatment:
Clinical evaluation: weight, ECOG-PS, vital signs (pulse, blood pressure, and body temperature),
Blood analysis: complete blood count including red blood cells, haemoglobin, haematocrit, lymphocytes, white blood cells differentials, platelets, blood electrolytes, bicarbonates, glycemia, protein, albumin, blood urea nitrogen, creatinine, creatinine clearance (CrCl) (by Modification of Diet in Renal Disease [MDRD]), calcium, magnesium, aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyl transpeptidase (G-GTP), conjugated and total bilirubin, alkaline phosphatase (ALP), thyroid stimulating hormone (TSH), lactate dehydrogenase (LDH), C-reactive protein (CRP), amylase, lipase
HIV, HCV, and HBV serology,
HIV viremia for HIV+ patients,
Serologic pregnancy test for women with childbearing potential,
Cardiac evaluation including electrocardiogram (ECG),
Blood sample n°1: one 6 ml EDTA tube and two 4 ml EDTA tubes (for plasma isolation and freezing) and six 6 ml EDTA tubes (for peripheral blood mononuclear cell [PBMC] storage that should be sent to Besançon Biomonitoring Platform at room temperature, cf. laboratory manual),
If all eligibility criteria are verified, randomization of the patient is done via the eCRF and a treatment arm is allocated to the patient.
Assessment before each chemotherapy cycle:
Including before cycle 1.
Blood analysis (platelets) done every week until 28 days after treatment induction and in case of fever or sign of infection, or evocative sign of an enterocolitis.
A time windows of +/− 3 days is allowed for chemotherapy cycle.
Clinical evaluation: weight, height, ECOG-PS, vital signs (pulse, blood pressure and body temperature),
Blood analysis (within 72 h prior the visit): complete blood count including red blood cells, haemoglobin, haematocrit, lymphocytes, white blood cells and differentials and platelets, blood electrolytes, bicarbonates, glycemia, protein, albumin, blood urea nitrogen, creatinine, CrCl (MDRD), calcium, magnesium, AST, ALT, G-GTP, conjugated and total bilirubin, ALP, TSH, LDH, CRP, amylase, lipase,
Serologic pregnancy test for women with childbearing potential before each treatment administration,
HRQoL evaluation with EORTC QLQ-C30 questionnaire: every 2 cycles (cf. Flowchart),
Adverse events report according to NCI-CTCAE guidelines v 4.03,
Serious adverse event/Adverse events of special interest report,
Concomitant treatment collection,
Assessment to be performed every 8 weeks from randomization to the end of treatment visit:
CT-scan every 2 months in order to determine the impact of DCF with/without atezolizumab treatment in each patient,
HIV viremia for HIV-positive patients.
Biomonitoring: Blood sample n°2, 3: to be taken respectively at 2 and 6 months from the date of randomization: one 6 ml EDTA tube and two 4 ml EDTA tubes (for plasma isolation and freezing), and six 6 ml EDTA tubes (for PBMC storage that should be sent to Besançon Biomonitoring Platform at room temperature, cf. laboratory manual).
End of trial
This study will end once all 99 recruited patients will complete 3 years of follow-up or die, whichever comes first. The patient can withdraw at any moment and this decision dose no need any justification. The patients will pursue the standard medical care.
Premature discontinuation of the study for the following reasons: patient’s decision, severe adverse event, protocol deviation, loss to follow-up, or death must be declared.
Monitoring and safety
An Independent Data and Safety Monitoring Committee (IDMC) represented by a multidisciplinary team, including physicians from relevant medical disciplines and biostatisticians, is implemented to:
✓ - Ensure the safety and well-being of the patients exposed to study treatment through an ongoing review of safety data,
✓ - Assure the highest integrity of the trial operation and performance,
✓ - Evaluate ongoing safety data to detect the possibility of an unfavourable early treatment risk.
In particular the combination of atezolizumab and mDCF will be closely followed.
IDMC’s responsibilities are:
✓ -To review safety data on an ongoing basis,
✓ - To recommend premature discontinuation if there is strong evidence that the investigational medicinal products are harming patients,
✓ - To make recommendations regarding modification of the study if there is strong evidence that such change would substantially contribute to the well-being of patients.
An IDMC meeting will take place within 10 days following an accomplishment of 2 months of treatment in the first 10 patients. Safety result and opinion of the committee will be sent to the ANSM (French Health Products Safety Agency) within 10 days after tolerance analyses. Additional meetings will be performed in any event of a signal that may affect the patient safety.
The IDMC will independently make its recommendations for continuation or termination of the trial to the appropriate Sponsor contact. The IDMC will maintain records of all its meetings and activities related to the study. These records will remain confidential until completion of the final analysis and only then will be forwarded to the Sponsor for appropriate archival.
The IDMC members will be aware of the data management and quality control procedures in order to assure timely compliance, accuracy, and completeness.
Discussion
Historically, prognosis of SCCA patients with advanced disease was poor with no prospectively validated chemotherapy regimen. The combinations of CF or CP were recommended based on retrospective analyses demonstrating the median PFS of 5.8–7.0 months and a 5-years overall survival (OS) rate less than 20% [6,7,8].
Docetaxel, a microtubule-stabilizing agent, exerts cytotoxic functions by blocking dividing cells in G2/M phase, leading to apoptosis. Docetaxel-based chemotherapy can modulate anti-tumour immune responses inducing calreticulin, a damage-associated molecular patterns associated to the immunogenic cell death [23].
Another possible effect of this treatment might be the depletion of immunosuppressive cells, sustaining the potential restoration of effective tumour immunity [24]. We have previously shown promising results with the addition of docetaxel to DCF in our Epitopes-HPV01 cohort study [9]. Four patients (out of eight) presented a long-lasting remission, providing a clinical rational for a prospective multicentre phase II Epitopes-HPV02 trial of 66 SCCA evaluable patients [25]. The trial confirmed the efficacy of this combination and settled the mDCF regimen as a new standard first-line chemotherapy [5]. Moreover, severe toxicities were halved with the mDCF regimen compared to the standard DCF. The immunomonitoring analysis of the Epitopes-HPV01 and Epitopes-HPV02 studies demonstrated that, i) MDSC play a major prognostic role in advanced SCCA patients in first-line, ii) DCF is capable to deplete MDSC and to induce an anti-tumour immune activity [5, 26]. Hence, considering those abilities and the favourable tolerance profile of mDCF, this regimen has been established as a good chemotherapy-based backbone candidate for a combination with immune checkpoint inhibitors [5].
After a long period without progress in advanced SCCA patients, there are now encouraging results with DCF in first-line and with CKIs in second or later lines with long-lasting responders, supporting the synergic effect between these treatments. Therefore, the evaluation of the DCF and an anti-PD1/L1 combination is warranted and will hopefully continue to improve prognosis of patients with this disease. The SCARCE study is the first trial to evaluate the combination of a chemotherapy regimen and CKI in advanced SCCA patients. These results might provide clinical evidence that CKI enhances long-term remission rate achieved by DCF-based chemotherapy and, if positive, could establish a new standard of care in this setting.
Availability of data and materials
Not applicable.
Abbreviations
- CRT:
-
Chemoradiotherapy
- CDDP:
-
Cisplatin
- CF:
-
Cisplatin and 5-fluorouracil
- CKI:
-
Checkpoint inhibitor
- CP:
-
Carboplatin and paclitaxel
- CR:
-
Complete response
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- DCF:
-
Docetaxel, cisplatin, and 5-fluorouracil
- ECOG-PS:
-
Eastern Cooperative Oncology Group-performance status
- eCRF:
-
Electronic case report form
- G-CSF:
-
Granulocyte colony-stimulating factor
- HPV:
-
Human papilloma virus
- HR:
-
Hazard ratio
- IDMC:
-
INDEPENDENT Data and Safety Monitoring Committee
- mDCF:
-
Modified DCF, docetaxel 40 mg/m2 day, CDDP 40 mg/m2 day and 5-FU at 1200 mg/m2/day for 2 days, every 2 weeks
- MDSC:
-
Myeloid-derived suppressor cells
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PD1/PD-L1:
-
Programmed cell death protein-1 immune checkpoint/ PD ligand
- PFS:
-
Progression-free survival
- QoL:
-
Health-related quality of life
- SCCA:
-
Squamous cell carcinoma of the anus
- sDCF:
-
Standard DCF - docetaxel 75 mg/m2/day, CDDP 75 mg/m2 and 5FU at 750 mg/m2/day for 5 days, every 3 weeks
- TF:
-
Timeframe
- Treg:
-
Regulatory T cells
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
Islami F, Ferlay J, Lortet-Tieulent J, Bray F, Jemal A. International trends in anal cancer incidence rates. Int J Epidemiol. 2016;39:276–15.
National Cancer Institute. SEER cancer statistics factsheets: anal cancer. seer.cancer.gov. 2016. Available from: http://seer.cancer.gov/statfacts/html/anus.html. [cited 2019 Nov 14].
Lépinoy A, Lescut N, Puyraveau M, Caubet M, Boustani J, Lakkis Z, et al. Evaluation of a 36 Gy elective node irradiation dose in anal cancer. Radiother Oncol. 2015;116:197–201.
Kim S, François E, André T, Samalin E, Jary M, El Hajbi F, et al. Docetaxel, cisplatin, and fluorouracil chemotherapy for metastatic or unresectable locally recurrent anal squamous cell carcinoma (epitopes-HPV02): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2018;19:1094–106.
Kim S, Meurisse A, Stouvenot M, Jary M, Nguyen T. Updated data of epitopes-HPV02 trial and external validation of efficacy of DCF in prospective epitopes-HPV01 study in advanced anal squamous cell carcinoma. Pooled analysis of 115 patients. Ann Oncol. 2019;30(Suppl 5):mdz246.012.
Baricevic I, He X, Chakrabarty B, Oliver AW, Bailey C, et al. High-sensitivity human papilloma virus genotyping reveals near universal positivity in anal squamous cell carcinoma: different implications for vaccine prevention and prognosis. Eur J Cancer. 2015;51:776–85.
Meulendijks D, Tomasoa NB, Dewit L, Smits PHM, Bakker R, et al. HPV-negative squamous cell carcinoma of the anal canal is unresponsive to standard treatment and frequently carries disruptive mutations in TP53. Br J Cancer. 2015;112:1358–66.
Kim S, Jary M, Mansi L, Benzidane B, Cazorla A, Demarchi M, et al. DCF (docetaxel, cisplatin and 5-fluorouracil) chemotherapy is a promising treatment for recurrent advanced squamous cell anal carcinoma. Ann Oncol. 2013;24:3045–50.
Cabel L, Jeannot E, Bieche I, Vacher S, Callens C, Bazire L, et al. Prognostic impact of residual HPV ctDNA detection after Chemoradiotherapy for anal squamous cell carcinoma. Clin Cancer Res. 2018;24:5767–71.
Bernard-Tessier A, Jeannot E, Guenat D, Debernardi A, Michel M. Clinical validity of HPV circulating tumor DNA in advanced anal carcinoma: an ancillary study to the epitopes-HPV02 trial. Clin Cancer Res. 2019;7:2109–15.
Morris VK. Circulating Tumor DNA in Advanced Anal Cancer: A Blood Biomarker Goes Viral. Clin Cancer Res. 2019;25:2030–2.
Rao S, Sclafani F, Eng C, Gronlie Guren M, Adams RA, et al. InterAACT: A multicentre open label randomised phase II advanced anal cancer trial of cisplatin (CDDP) plus 5-fluorouracil (5-FU) vs carboplatin (C) plus weekly paclitaxel (P) in patients (pts) with inoperable locally recurrent (ILR) or metastatic treatment na ̈ıve disease - An International Rare Cancers Initiative (IRCI) trial. Ann Oncol. 2018;29(Suppl 8):mdy424.022.
Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–41.
Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck Cancer. Cancer Res. 2013;73:128–38.
Oliveira-Costa JP, de Carvalho AF, da Silveira da GG, Amaya P, Wu Y, Park KJ, Gigliola MP, et al. Gene expression patterns through oral squamous cell carcinoma development: PD-L1 expression in primary tumor and circulating tumor cells. Oncotarget. 2015;6:20902–20920.
Morris VK, Salem M, Nimeiri H, Iqbal S, Singh P, Ciombor K, et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:446–53.
Ott PA, Piha-Paul SA, Munster P, Pishvaian MJ, van Brummelen EMJ, Cohen RB, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann Oncol. 2017;28:1036–41.
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–301.
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung Cancer. N Engl J Med. 2018;378:2078–92.
Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line Atezolizumab plus chemotherapy in extensive-stage small-cell lung Cancer. N Engl J Med. 2018;379:2220–9.
Conroy T, Mercier M, Bonneterre J, Luporsi E, Lefebvre JL, Lapeyre M, et al. French version of FACT-G: validation and comparison with other cancer-specific instruments. Eur J Cancer. 2004;40:2243–52.
Bezu L, Gomes-de-Silva LC, Dewitte H, Breckpot K, Fucikova J, Spisek R, et al. Combinatorial strategies for the induction of immunogenic cell death. Front Immunol. Frontiers. 2015;6:187.
Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714.
Kim S, Jary M, André T, Vendrely V, Buecher B, François E, et al. Docetaxel, Cisplatin, and 5-fluorouracil (DCF) chemotherapy in the treatment of metastatic or unresectable locally recurrent anal squamous cell carcinoma: a phase II study of French interdisciplinary GERCOR and FFCD groups (epitopes-HPV02 study). BMC Cancer. 2017;17:574.
Borg C, Spehner L, Kroemer M, Kim S, Jary M, et al. Myeloid derived suppressor cells but not regulatory T cells are associated with adaptive immunity and clinical outcomes in anal squamous cell carcinoma. Ann Oncol. 2019;30(Suppl 5):mdz268.030.
Acknowledgements
The authors would like to thank Magdalena Benetkiewicz and Guadalupe Inés Tizón for editing assistance. We would like to acknowledge Dr. Marie-Line Garcia-Larnicol and all GERCOR members for their implications in conducting the study.
Trial status
The first patient was included in June 26, 2018 after all legal approvals required. The current recruitment rate is as scheduled.
Funding
This investigator-initiated trial is sponsored by GERCOR (France) with funding provided by Roche France. The funder has no role in study design, data collection, analysis, and interpretation, or preparation of the manuscript and decision to publish.
Author information
Authors and Affiliations
Contributions
Conception and design: SK, MJ, F-CB, TA, CT, MJac, MR-P, LS, VV, DV, CB. Protocol writing: SK, MJ, MJac, MR-P, DV, CB. Methods: SK, DV, CB. Study promotion: SK, TA, CT, VV, CB. Patients inclusion and materials provision: SK, BB, TA, MJ, F-CB, FG, EF, JT, DS, CD, JD, ES, AP, NB-H, OB, DT, LD, FE-H, CB. Manuscript writing: SK, MJ, MJac, MR-P, LS, DV, CB. Critical lecture and final approval of the manuscript: all authors
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This trial is carried out in accordance with Public Health Act 2004–806 of 9 August 2004 concerning biomedical research, Decree No. 2006–477 of 26.04.04, 2006 amending Chapter I of Title II of Book 1 of the first part of the Public Health Code concerning biomedical research and the decrees in force. The Investigator and the promoter undertake to follow the rules of good clinical practice (BPC) of biomedical research on medicinal products for human use, mentioned in Article L. 1121–3 of the Public Health Code and Order of 23 April 2004. The current study is conducted in accordance with globally accepted standards of Good Clinical Practice (ICH-E6), European Directive 2001/20/EC, and the revised version of the Declaration of Helsinki set out in the European Directive, as well as with the Certificate Service Providers (CSP) specific to France. Before the study start, each patient is informed by the Investigator, in writing as well as verbally, about the nature and implications of the proposed study, and chiefly of the possible benefits and risks for their health, having this notice been previously approved by the Committee for the Protection of Persons. Patients have at least 48 h for reflexion on the topic. Consequently, patients document their approval by signing an informed consent form before any intervention or procedure specified in the protocol. Both, the Investigator and the patient, sign the consent form, and each part saves one example. The study was approved by the independent Committee for Protection of Persons “Sub Méditerranée IV” (April 10, 2018) and by the French Health Products Safety Agency (April 16, 2018).
For biomedical research in France: The Sponsor attests his conformity regarding the Personal Data Protection French requirements with the current version of “Méthodologie de Réference MR001”.
In accordance with the law “Informatique et Libertés” (Law 78–17 of 6 January 1978 amended by Act 2004–801 of 6 August 2004), the type of data collected concerning patients participating in this study will be sent to the Data Protection Supervisory Authority (CNIL). Patients will be informed of this procedure and of their right of access and rectification of the data concerning them.
A processing of the personal data will be implemented to allow analysis of the results, under conditions ensuring their confidentiality. These data may, under conditions guaranteeing their confidentiality, be transmitted to the French or foreign health authorities. The patient has a right of access and rectification. The patient also has the right to object to the transmission of his/her personal data used in this study. These rights are exercised with the investigating physician who follows the patient in the course of the study and who knows the patient identity.
Approval by the Méditerranée-4 Committee for Protection of Persons on May 15, 2018 and by the French Health Products Safety Agency on April 16, 2018.
Protocol version: 2.0, February 5th 2019.
Consent for publication
Not applicable.
Competing interests
Genentech provides atezolizumab and Roche France funds the study.
The authors declare no other competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, S., Buecher, B., André, T. et al. Atezolizumab plus modified docetaxel-cisplatin-5-fluorouracil (mDCF) regimen versus mDCF in patients with metastatic or unresectable locally advanced recurrent anal squamous cell carcinoma: a randomized, non-comparative phase II SCARCE GERCOR trial. BMC Cancer 20, 352 (2020). https://doi.org/10.1186/s12885-020-06841-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-020-06841-1





