Abstract
Background
The 5-year survival for patients with esophageal carcinoma remains poor despite neoadjuvant therapy and surgery. The eighth American Joint Committee on Cancer (AJCC) staging, based on the neoadjuvant treated TNM (ypTNM) stage of the resection specimen, is used for prognosis. Tumor characteristics such as tumor grade, subtype of adenocarcinoma, and tumor regression scores are not included in this classification. This study aimed to determine the impact of these tumor characteristics on overall survival (OS) and disease-free survival (DFS).
Methods
This retrospective cohort study included 228 patients with esophageal adenocarcinoma. Tumor regression was determined by the Mandard tumor regression (MTR) score. Subtype and grade of adenocarcinoma were confirmed using either the preoperative biopsy or residual tumor tissue after surgery. The MTR was modified to a three-tier classification. The study classified MTR 1 and 2 in one group as a “major response,” with MTR 4 and 5 classified in one group as a “minimal response.”
Results
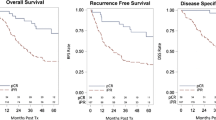
The median follow-up period was 2.1 years. Combining MTR with AJCC staging did not improve the prognostic value for the prediction of OS. However, the multivariate analysis showed that the prognostic value of AJCC staging for DFS was improved by adding the three-tiered MTR (odds ratio for MTR4+5: 2.46; 95 % confidence interval, 1.07–5.67). Grade or subtype correlated with neither OS nor DFS in the univariate analyses and did not improve the prognostic value of the AJCC staging.
Conclusion
Neither adenocarcinoma subtype nor grade influenced OS or DFS. However, the eighth AJCC staging combined with a three-tier MTR provided a better prognostic tool for DFS in esophageal adenocarcinoma treated with esophagectomy after neoadjuvant chemoradiotherapy.

Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
van Putten M, de Vos-Geelen J, Nieuwenhuijzen GAP, et al. Long-term survival improvement in oesophageal cancer in the Netherlands. Eur J Cancer. 2018;94:138–47. https://doi.org/10.1016/j.ejca.2018.02.025.
Khan OA, Alexiou C, Soomro I, Duffy JP, Morgan WE, Beggs FD. Pathological determinants of survival in node-negative oesophageal cancer. Br J Surg. 2004;91:1586–91. https://doi.org/10.1002/bjs.4778.
Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomized controlled trial. Lancet Oncol. 2015;16:1090–8. https://doi.org/10.1016/S1470-2045(15)00040-6.
Noble F, Nolan L, Bateman AC, et al. Refining pathological evaluation of neoadjuvant therapy for adenocarcinoma of the esophagus. World J Gastroenterol. 2013;19:9282–93. https://doi.org/10.3748/wjg.v19.i48.9282.
Tomasello G, Ghidini M, Barni S, Passalacqua R, Petrelli F. Overview of different available chemotherapy regimens combined with radiotherapy for the neoadjuvant and definitive treatment of esophageal cancer. Expert Rev Clin Pharmacol. 2017;10:649–60. https://doi.org/10.1080/17512433.2017.1313112.
Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6:119–30. https://doi.org/10.21037/acs.2017.03.14.
Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma: an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. https://doi.org/10.1111/apm.1965.64.1.31.
Vošmik M, Laco J, Sirák I, et al. Histopathologic features are more important prognostic factors than primary tumour location in gastro-oesophageal adenocarcinoma treated with preoperative chemoradiation and surgery. Pathol Oncol Res. 2018;24:373–83. https://doi.org/10.1007/s12253-017-0253-z.
Spoerl S, Novotny A, Al-Batran SE, et al. Histopathological regression predicts treatment outcome in locally advanced esophagogastric adenocarcinoma. Eur J Cancer. 2018;90:26–33. https://doi.org/10.1016/j.ejca.2017.11.020.
van der Kaaij RT, Snaebjornsson P, Voncken FEM, et al. The prognostic and potentially predictive value of the Laurén classification in oesophageal adenocarcinoma. Eur J Cancer. 2017;76:27–35. https://doi.org/10.1016/j.ejca.2017.01.031.
van der Kaaij RT, Koemans WJ, van Putten M, et al. A population-based study on intestinal and diffuse type adenocarcinoma of the oesophagus and stomach in the Netherlands between 1989 and 2015. Eur J Cancer. 2020;130:23–31. https://doi.org/10.1016/j.ejca.2020.02.017.
Kim T, Grobmyer SR, Smith R, et al. Esophageal cancer: the five year survivors. J Surg Oncol. 2011;103:179–83. https://doi.org/10.1002/jso.21784.
Hou X, Gu YK, Liu XW, et al. The impact of tumor cell differentiation on survival of patients with resectable esophageal squamous cell carcinomas. Ann Surg Oncol. 2015;22:1008–14. https://doi.org/10.1245/s10434-014-4067-x.
Mandard A-M, Dalibard F, Mandard J-C, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–6. https://doi.org/10.1002/1097-0142(19940601)73:11%3c2680::AID-CNCR2820731105%3e3.0.CO;2-C.
Karamitopoulou E, Thies S, Zlobec I, et al. Assessment of tumor regression of esophageal adenocarcinomas after neoadjuvant chemotherapy. Am J Surg Pathol. 2014;38:1551–6. https://doi.org/10.1097/pas.0000000000000255.
Hölscher AH, Drebber U, Schmidt H, Bollschweiler E. Prognostic classification of histopathologic response to neoadjuvant therapy in esophageal adenocarcinoma. Ann Surg. 2014;260:779–85. https://doi.org/10.1097/SLA.0000000000000964.
Puetz K, Bollschweiler E, Semrau R, Mönig SP, Hölscher AH, Drebber U. Neoadjuvant chemoradiation for patients with advanced oesophageal cancer: which response grading system best impacts prognostic discrimination? Histopathology. 2019;74:731–43. https://doi.org/10.1111/his.13811.
Zhu Y, Sun Y, Hu S, et al. Comparison of five tumor regression grading systems for gastric adenocarcinoma after neoadjuvant chemotherapy: a retrospective study of 192 cases from National Cancer Center in China. BMC Gastroenterol. 2017;17:1–18. https://doi.org/10.1186/s12876-017-0598-5.
Donohoe CL, O’Farrell NJ, Grant T, et al. Classification of pathologic response to neoadjuvant therapy in esophageal and junctional cancer: assessment of existing measures and proposal of a novel 3-point standard. Ann Surg. 2013;258:784–92. https://doi.org/10.1097/SLA.0b013e3182a66588.
Maag-darm-leverartsen V Van. Oesofaguscarcinoom Inhoudsopgave. Published online 2010:1–12. Accessed 8 July 2021.
Ryan R, Gibbons D, Hyland JMP, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47:141–6. https://doi.org/10.1111/j.1365-2559.2005.02176.x.
Sarbia M. The histological appearance of oesophageal adenocarcinoma: an analysis based on 215 resection specimens. Virchows Arch. 2006;448:532–8. https://doi.org/10.1007/s00428-006-0168-7.
Polkowski W, Van Sandick JW, Offerhaus GJA, et al. Prognostic value of Lauren classification and c-erbB-2 oncogene overexpression in adenocarcinoma of the esophagus and gastroesophageal junction. Ann Surg Oncol. 1999;6:290–7. https://doi.org/10.1007/s10434-999-0290-2.
van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. https://doi.org/10.1056/nejmoa1112088.
Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–92. https://doi.org/10.1016/S1470-2045(11)70142-5.
Zhao X, Ren Y, Hu Y, Cui N, Wang X, Cui Y. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or the gastroesophageal junction: a meta-analysis based on clinical trials. PloS One. 2018;13:1–19. https://doi.org/10.1371/journal.pone.0202185.
Deng HY, Wang WP, Wang YC, et al. Neoadjuvant chemoradiotherapy or chemotherapy? A comprehensive systematic review and meta-analysis of the options for neoadjuvant therapy for treating oesophageal cancer. Eur J Cardiothorac Surg. 2017;51:421–31. https://doi.org/10.1093/ejcts/ezw315.
Von Döbeln GA, Klevebro F, Jacobsen AB, et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: long-term results of a randomized clinical trial. Dis Esophagus. 2019;32:1–11. https://doi.org/10.1093/dote/doy078.
van Hootegem SJM, Smithers BM, Gotley DC, et al. The impact of signet ring cell differentiation on outcome in patients with esophageal and gastroesophageal junction adenocarcinoma. Ann Surg Oncol. 2019;26:2375–84. https://doi.org/10.1245/s10434-019-07322-x.
Hagens E, Tukanova K, Jamel S, et al. Prognostic relevance of lymph node regression on survival in esophageal cancer: a systematic review and meta-analysis. Published online 2021:1–11. https://doi.org/10.1093/dote/doab021.
Smyth EC, Fassan M, Cunningham D, et al. Effect of pathologic tumor response and nodal status on survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. Science. 2016. https://doi.org/10.1200/JCO.2015.65.7692.
Depypere L, Moons J, Lerut T, et al. Neoadjuvant chemoradiation treatment followed by surgery for esophageal cancer: there is much more than the Mandard tumor regression score. Acta Chir Belg. 2016;116:149–55. https://doi.org/10.1080/00015458.2016.1212500.
Verlato G, Zanoni A, Tomezzoli A, et al. Response to induction therapy in oesophageal and cardia carcinoma using Mandard tumour regression grade or size of residual foci. Br J Surg. 2010;97:719–25. https://doi.org/10.1002/bjs.6949.
Yuan Y, Ma G, Hu X, Huang Q. Evaluating the eighth edition TNM staging system for esophageal cancer among patients receiving neoadjuvant therapy: a SEER study. Cancer Med. 2020;9:4648–55. https://doi.org/10.1002/cam4.2997.
Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–7. https://doi.org/10.1200/JCO.2009.22.2083.
Depypere L, Lerut T, Moons J, et al. Isolated local recurrence or solitary solid organ metastasis after esophagectomy for cancer is not the end of the road. Dis Esophagus. 2017;30:1–8. https://doi.org/10.1111/dote.12508.
Lou F, Sima CS, Adusumilli PS, et al. Esophageal cancer recurrence patterns and implications for surveillance. J Thorac Oncol. 2013;8:1558–62. https://doi.org/10.1097/01.JTO.0000437420.38972.fb.
Burt BM, Groth SS, Sada YH, et al. Utility of adjuvant chemotherapy after neoadjuvant chemoradiation and esophagectomy for esophageal cancer. Ann Surg. 2017;266:297–304. https://doi.org/10.1097/SLA.0000000000001954.
Mokdad AA, Yopp AC, Polanco PM, et al. Adjuvant chemotherapy vs postoperative observation following preoperative chemoradiotherapy and resection in gastroesophageal cancer a propensity score-matched analysis. JAMA Oncol. 2018;4:31–8. https://doi.org/10.1001/jamaoncol.2017.2805.
Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384:1191–203. https://doi.org/10.1056/nejmoa2032125.
Turgeman I, Ben-Aharon I. Evolving treatment paradigms in esophageal cancer. Ann Transl Med. 2021;9:903–918. https://doi.org/10.21037/atm.2020.03.110.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Crull, D.J., Hogenes, M.C.H., Hoekstra, R. et al. The Impact of Tumor Regression on Prognosis After Neoadjuvant Chemoradiotherapy in Surgically Treated Esophageal Adenocarcinoma. Ann Surg Oncol 29, 3658–3666 (2022). https://doi.org/10.1245/s10434-022-11336-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11336-3