Abstract
Background
The American College of Surgeons Oncology Group (ACOSOG) Z1071 and Sentinel Neoadjuvant (SENTINA) trials of sentinel node biopsy for node-positive breast cancer treated with neoadjuvant chemotherapy (NAC) demonstrated false-negative rates that varied on the basis of surgical technique. This study evaluated trends in axillary operations before and after publication of these trials.
Methods
This study analyzed patients from National Cancer Database (NCDB) with clinical T0 through T4, N1 and N2, M0 breast cancer who received NAC from 1 January 2012 to 31 December 2015 and sentinel lymph node biopsy (SNB) or axillary lymph node dissection (ALND). The patients were divided into the following groups: SNB, ALND, and (SNB + ALND).
Results
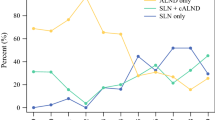
Of the 32,036 evaluable patients identified in this study. 5565 had SNB, 19,930 had ALND, and 6541 had SNB + ALND. Compared with the ALND group, the SNB group was younger, had more invasive ductal cancers, and had lower clinical T- and N-stage disease (p < 0.001). The patients in the SNB group had a higher rate of estrogen receptor-positive and triple-negative breast cancers, but a lower rate of human epidermal growth factor receptor 2 (HER2)-positive cancer (p < 0.001). The nodal pathologic complete response (PCR) rate, defined as no residual invasive cancer, was 66.5% in the SNB group and 33.1% in the ALND group. Since 2013, the rate of ALND has decreased from 88.7 to 77.1% in both community and academic institutions (p < 0.001).
Conclusion
Since publication of the ACOSOG Z1071 and SENTINA trials, the national rates of ALND in node positive breast cancer treated with NAC have decreased despite reported false-negative SNB rates and lack of prospective outcome data regarding the oncologic safety of ALND omission.

Similar content being viewed by others
References
Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer—The ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455–61.
Mittendorf EA, Caudle AS, Yang W, et al. Implementation of the American College of Surgeons Oncology Group Z1071 trial data in clinical practice: Is there a way forward for sentinel lymph node dissection in clinically node-posiitve breast cancer patients treated with neoadjuvant chemotherapy? Ann Surg Oncol. 2014;21:2468–73.
Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0–T4, N1–N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance). Ann Surg Oncol. 2016;263:802–7.
Boughey JC, Ballman KV, McCall LM, et al. Tumor biology and response to chemotherapy impact breast cancer-specific survival in node-positive breast cancer patients treated with neoadjuvant chemotherapy: long-term follow-up from ACOSOG Z1071 (Alliance). Ann Surg Oncol. 2017;266:667–76.
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicenter cohort study. Lancet Oncol. 2013;14:609–18.
Boileau JF, Boirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-posiitve breast cancer: the SN FNAC study. J Clin Oncol. 2015;33:258–64.
Caudle AS, Bedrosian I, Milton DR, DeSnyder SM, Kuerer HM, Hunt KK, Mittendorf EA. Use of sentinal lymph node dissection after neoadjuvant chemotherapy in patients with node-positive breast cancer at diagnosis: practice patterns of American Society of Breast Surgeons members. Ann Surg Oncol. 2017;24:2925–34.
Palmer JAV, Flippo-Morton T, Walsh KK, Gusic LH, Sarantou T, Robinson MM, White RL Jr. Application of ACOSOG Z1071: effect of results on patient care and surgical decision making. Clin Breast Cancer. 2018;18:270–5.
Acknowledgment
The authors thank the Fashion Footwear Charitable Foundation of New York, Inc.; The Margie and Robert E. Petersen Foundation Associates for Breast and Prostate Cancer Studies; and Linda and Jim Lippman for their contributions to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Srour, M.K., Tseng, J., Luu, M. et al. Patterns in the Use of Axillary Operations for Patients with Node-Positive Breast Cancer After Neoadjuvant Chemotherapy: A National Cancer Database (NCDB) Analysis. Ann Surg Oncol 26, 3305–3311 (2019). https://doi.org/10.1245/s10434-019-07540-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07540-3