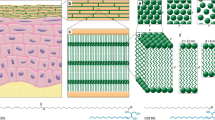
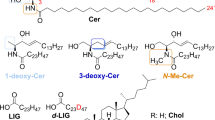
Abstract
Disrupted skin barrier, one of the severe attributes of inflammatory skin diseases, is caused by lower content and pathological changes of lipids in the uppermost skin layer—stratum corneum (SC). Restoring skin barrier with native skin lipids, especially ceramides (Cers), appears to be a promising therapy with minimum side effects. For testing the efficiency of these formulations, suitable in vitro models of the skin with disrupted barriers are needed. For the similarity with the human tissue, our models were based on the pig ear skin. Three different ways of skin barrier disruption were tested and compared: tape stripping, lipid extraction with organic solvents, and barrier disruption by sodium lauryl sulfate. The level of barrier disruption was investigated by permeation studies, and parameters of each method were modified to reach significant changes between the non-disrupted skin and our model. Fourier transform infrared (FTIR) spectroscopy was employed to elucidate the changes of the skin permeability on the molecular scale. Further, the potential of the developed models to be restored by skin barrier repairing agents was evaluated by the same techniques. We observed a significant decrease in permeation characteristics through our in vitro models treated with the lipid mixtures compared to the untreated damaged skin, which implied that the skin barrier was substantially restored. Taken together, the results suggest that our in vitro models are suitable for the screening of potential barrier repairing agents.
Graphical Abstract




Similar content being viewed by others
Abbreviations
- AD:
-
Atopic dermatitis
- ATR:
-
Attenuated total reflectance
- BR:
-
Barrier recovery percent
- Cer:
-
Ceramide
- Cer AP:
-
N-2-hydroxystearoyl phytosphingosine
- Cer NP:
-
N-stearoyl phytosphingosine
- Chol:
-
Cholesterol
- CS:
-
Cholesterol sulfate
- DR:
-
Disrupting ratio
- DSC:
-
Differential scanning calorimetry
- FFA:
-
Free fatty acids
- FTIR:
-
Fourrier transform infrared
- IND:
-
Indomethacin
- IR:
-
Infrared
- HPLC:
-
High-performance liquid chromatography
- PBS:
-
Phosphate buffer saline
- SA:
-
Stearic acid
- SC:
-
Stratum corneum
- SCORAD:
-
Severity scoring of atopic dermatitis
- SEM:
-
Standard error of the mean
- SLS:
-
Sodium lauryl sulfate
- TCS:
-
Topical corticosteroids
- TEWL:
-
Transepidermal water loss
- TH:
-
Theophylline
References
Menon GK. New insights into skin structure: scratching the surface. Adv Drug Del Rev. 2002;54(Supplement):S3–17.
Fartasch M. The nature of the epidermal barrier: structural aspects. Adv Drug Deliv Rev. 1996;18(3):273–82.
Menon GK, Cleary GW, Lane ME. The structure and function of the stratum corneum. Int J Pharm. 2012;435(1):3–9.
Yardley HJ, Summerly R. Lipid composition and metabolism in normal and diseased epidermis. Pharmacol Ther. 1981;13(2):357–83.
Schurer NY, Elias PM. The biochemistry and function of stratum corneum lipids. In: Elias PM, editor. Advances in Lipid Research. 24: Elsevier; 1991. p. 27–56.
Lampe MA, Burlingame AL, Whitney J, Williams ML, Brown BE, Roitman E, et al. Human stratum corneum lipids: characterization and regional variations. J Lipid Res. 1983;24(2):120–30.
Feingold KR, Elias PM. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim Biophys Acta (BBA) - Mol Cell Biol Lipids. 2014;1841(3):280–94.
Bouwstra JA, Ponec M. The skin barrier in healthy and diseased state. Biochim Biophys Acta, Biomembr. 2006;1758(12):2080–95.
van Smeden J, Janssens M, Gooris GS, Bouwstra JA. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim Biophys Acta, Mol Cell Biol Lipids. 2014;1841(3):295–313.
Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population–based study. J Allergy Clin Immunol. 2013;132(5):1132–8.
Wolf R, Orion E, Ruocco E, Ruocco V. Abnormal epidermal barrier in the pathogenesis of psoriasis. Clin Dermatol. 2012;30(3):323–8.
van Smeden J, Bouwstra JA. Stratum corneum lipids: their role for the skin barrier function in healthy subjects and atopic dermatitis patients. Curr Probl Dermatol. 2016;49:8–26.
Borodzicz S, Rudnicka L, Mirowska-Guzel D, Cudnoch-Jedrzejewska A. The role of epidermal sphingolipids in dermatologic diseases. Lipids Health Dis. 2016;15(1):13.
Callen J, Chamlin S, Eichenfield LF, Ellis C, Girardi M, Goldfarb M, et al. A systematic review of the safety of topical therapies for atopic dermatitis. Br J Dermatol. 2007;156(2):203–21.
Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54(1):1–15.
Arkwright PD, Motala C, Subramanian H, Spergel J, Schneider LC, Wollenberg A. Management of difficult-to-treat atopic dermatitis. J Allergy Clin Immunol Pract. 2013;1(2):142–51.
Roekevisch E, Leeflang MMG, Schram ME, Campbell LE, Irwin McLean WH, Kezic S, et al. Patients with atopic dermatitis with filaggrin loss-of-function mutations show good but lower responses to immunosuppressive treatment. Br J Dermatol. 2017;177(6):1745–6.
Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–32.
Berkers T, Visscher D, Gooris GS, Bouwstra JA. Topically applied ceramides interact with the stratum corneum lipid matrix in compromised ex vivo skin. Pharm Res. 2018;35(3):48.
Chamlin SL, Frieden IJ, Fowler A, Williams M, Kao J, Sheu M, et al. Ceramide-dominant, barrier-repair lipids improve childhood atopic dermatitis. Arch Dermatol. 2001;137(8):1110–2.
Elias PM. Lipid abnormalities and lipid-based repair strategies in atopic dermatitis. Biochim Biophys Acta (BBA) - Mol Cell Biol Lipids. 2014;1841(3):323–30.
Draelos ZD. New treatments for restoring impaired epidermal barrier permeability: skin barrier repair creams. Clin Dermatol. 2012;30(3):345–8.
Sugarman JL, Parish LC. Efficacy of a lipid-based barrier repair formulation in moderate-to-severe pediatric atopic dermatitis. J Drugs Dermatol. 2009;8(12):1106–11.
Vovesná A, Zhigunov A, Balouch M, Zbytovská J. Ceramide liposomes for skin barrier recovery: a novel formulation based on natural skin lipids. Int J Pharm. 2021;596:120264.
Bashir SJ, Chew A-L, Anigbogu A, Dreher F, Maibach HI. Physical and physiological effects of stratum corneum tape stripping. Skin Res Technol. 2001;7(1):40–8.
Yoshizawa Y, Kitamura K, Kawana S, Maibach HI. Water, salts and skin barrier of normal skin. Skin Res Technol. 2003;9(1):31–3.
Man M-Q, Feingold KR, Thornfeldt CR, Elias PM. Optimization of physiological lipid mixtures for barrier repair. J Invest Dermatol. 1996;106(5):1096–101.
Bissonnette R, Maari C, Provost N, Bolduc C, Nigen S, Rougier A, et al. A double-blind study of tolerance and efficacy of a new urea-containing moisturizer in patients with atopic dermatitis. J Cosmet Dermatol. 2010;9(1):16–21.
Seghers AC, Cai SC, Ho MSL, Giam YC, Tan L, Grönhagen CM, et al. Evaluation of a pseudoceramide moisturizer in patients with mild-to-moderate atopic dermatitis. Dermatol Ther. 2014;4(1):83–92.
Andrews S, Lee JW, Prausnitz M. Recovery of skin barrier after stratum corneum removal by microdermabrasion. AAPS PharmSciTech. 2011;12(4):1393–400.
Welss T, Basketter DA, Schröder KR. In vitro skin irritation: facts and future. State of the art review of mechanisms and models. Toxicol in Vitro. 2004;18(3):231–43.
Faller C, Bracher M, Dami N, Roguet R. Predictive ability of reconstructed human epidermis equivalents for the assessment of skin irritation of cosmetics☆☆This study is part of the European project SMT4-CT 97–2174: “Testing and improvement of reconstructed skin kits in order to elaborate European standards”. Toxicol in Vitro. 2002;16(5):557–72.
Schäfer-Korting M, Mahmoud A, Borgia SL, Brüggener B, Kleuser B, Schreiber S, et al. Reconstructed epidermis and full-thickness skin for absorption testing: influence of the vehicles used on steroid permeation. Altern Lab Anim. 2008;36(4):441–52.
Sekkat N, Kalia YN, Guy RH. Biophysical study of porcine ear skin in vitro and its comparison to human skin in vivo. J Pharm Sci. 2002;91(11):2376–81.
Barbero AM, Frasch HF. Pig and guinea pig skin as surrogates for human in vitro penetration studies: a quantitative review. Toxicol in Vitro. 2009;23(1):1–13.
Danso MO, Berkers T, Mieremet A, Hausil F, Bouwstra JA. An ex vivo human skin model for studying skin barrier repair. Exp Dermatol. 2015;24(1):48–54.
Abrams K, Harvell JD, Shriner D, Wertz P, Maibach H, Maibach HI, et al. Effect of organic solvents on in vitro human skin water barrier function. J Invest Dermatol. 1993;101(4):609–13.
Barba C, Alonso C, Martí M, Manich A, Coderch L. Skin barrier modification with organic solvents. Biochim Biophys Acta (BBA)- Biomembr. 2016;1858(8):1935–43.
Wolf R, Parish LC. Effect of soaps and detergents on epidermal barrier function. Clin Dermatol. 2012;30(3):297–300.
Klang V, Schwarz JC, Lenobel B, Nadj M, Auböck J, Wolzt M, et al. In vitro vs. in vivo tape stripping: validation of the porcine ear model and penetration assessment of novel sucrose stearate emulsions. Eur J Pharm Biopharm. 2012;80(3):604–14.
Lademann J, Jacobi U, Surber C, Weigmann HJ, Fluhr JW. The tape stripping procedure – evaluation of some critical parameters. Eur J Pharm Biopharm. 2009;72(2):317–23.
Tsai JC, Sheu HM, Hung PL, Cheng CL. Effect of barrier disruption by acetone treatment on the permeability of compounds with various lipophilicities: implications for the permeability of compromised skin. J Pharm Sci. 2001;90(9):1242–54.
Löffler H, Dreher F, Maibach HI. Stratum corneum adhesive tape stripping: influence of anatomical site, application pressure, duration and removal. Br J Dermatol. 2004;151(4):746–52.
Rissmann R, Oudshoorn MHM, Hennink WE, Ponec M, Bouwstra JA. Skin barrier disruption by acetone: observations in a hairless mouse skin model. Arch Dermatol Res. 2009;301(8):609–13.
Fluhr JW, Feingold KR, Elias PM. Transepidermal water loss reflects permeability barrier status: validation in human and rodent in vivo and ex vivo models. Exp Dermatol. 2006;15(7):483–92.
Siewert M, Dressman J, Brown CK, Shah VP, Aiache J-M, Aoyagi N, et al. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSciTech. 2003;4(1):43–52.
Chilcott RP, Dalton CH, Emmanuel AJ, Allen CE, Bradley ST. Transepidermal water loss does not correlate with skin barrier function in vitro. J Invest Dermatol. 2002;118(5):871–5.
Levin J, Maibach H. The correlation between transepidermal water loss and percutaneous absorption: an overview. J Control Release. 2005;103(2):291–9.
Čuříková BA, Procházková K, Filková B, Diblíková P, Svoboda J, Kováčik A, et al. Simplified stratum corneum model membranes for studying the effects of permeation enhancers. Int J Pharm. 2017;534(1):287–96.
Kopecna M, Machacek M, Prchalova E, Stepanek P, Drasar P, Kotora M, et al. Dodecyl amino glucoside enhances transdermal and topical drug delivery via reversible interaction with skin barrier lipids. Pharm Res. 2017;34(3):640–53.
Lasch J, Weissig V, Brandl MJLapa. Preparation of liposomes. 2003;2:24-5.
Sochorová M, Staňková K, Pullmannová P, Kováčik A, Zbytovská J, Vávrová K. Permeability barrier and microstructure of skin lipid membrane models of impaired glucosylceramide processing. Sci Rep. 2017;7(1).
Gysler A, Kleuser B, Sippl W, Lange K, Korting HC, Höltje H-D, et al. Skin penetration and metabolism of topical glucocorticoids in reconstructed epidermis and in excised human skin. Pharm Res. 1999;16(9):1386–91.
Carrer V, Guzmán B, Martí M, Alonso C, Coderch L. Lanolin-based synthetic membranes as percutaneous absorption models for transdermal drug delivery. Pharmaceutics. 2018;10(3):73.
Kopečná M, Macháček M, Prchalová E, Štěpánek P, Drašar P, Kotora M, et al. Galactosyl pentadecene reversibly enhances transdermal and topical drug delivery. Pharm Res. 2017;34(10):2097–108.
Buraczewska I, Berne B, Lindberg M, Törmä H, Lodén MJBJoD. Changes in skin barrier function following long‐term treatment with moisturizers, a randomized controlled trial. 2007;156(3):492–8.
Yang L, Mao-QlAng M, Taljebini M, Elias PM, Feingold KR. Topical stratum corneum lipids accelerate barrier repair after tape stripping, solvent treatment and some but not all types of detergent treatment. Br J Dermatol. 1995;133(5):679–85.
Visscher MO, Hoath SB, Conroy E, Wickett RR. Effect of semipermeable membranes on skin barrier repair following tape stripping. Arch Dermatol Res. 2001;293(10):491–9.
Hathout RM, Mansour S, Mortada ND, Geneidi AS, Guy RH. Uptake of microemulsion components into the stratum corneum and their molecular effects on skin barrier function. Mol Pharm. 2010;7(4):1266–73.
Yu G, Zhang G, Flach C, Mendelsohn R. Vibrational spectroscopy and microscopic imaging: novel approaches for comparing barrier physical properties in native and human skin equivalents. J Biomed Opt. 2012;18(6):061207.
Clancy MJ, Corish J, Corrigan OI. A comparison of the effects of electrical current and penetration enhancers on the properties of human skin using spectroscopie (FTIR) and calorimetric (DSC) methods. Int J Pharm. 1994;105(1):47–56.
Jacobi U, Weigmann HJ, Ulrich J, Sterry W, Lademann J. Estimation of the relative stratum corneum amount removed by tape stripping. Skin Res Technol. 2005;11(2):91–6.
Kalia YN, Alberti I, Sekkat N, Curdy C, Naik A, Guy RH. Normalization of stratum corneum barrier function and transepidermal water loss in vivo. Pharm Res. 2000;17(9):1148–50.
Gao Y, Wang X, Chen S, Li S, Liu X. Acute skin barrier disruption with repeated tape stripping: an in vivo model for damage skin barrier. Skin Res Technol. 2013;19(2):162–8.
Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(1):911–7.
Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. 1998.
Mitragotri S. Modeling skin permeability to hydrophilic and hydrophobic solutes based on four permeation pathways. J Control Rel. 2003;86(1):69–92.
Johnson ME, Berk DA, Blankschtein D, Golan DE, Jain RK, Langer RS. Lateral diffusion of small compounds in human stratum corneum and model lipid bilayer systems. Biophys J. 1996;71(5):2656–68.
Jacobi U, Taube H, Schäfer UF, Sterry W, Lademann J. Comparison of four different in vitro systems to study the reservoir capacity of the stratum corneum. J Control Release. 2005;103(1):61–71.
Rougier A, Rallis M, Krien P, Lotte C. In vivo percutaneous absorption: a key role for stratum corneum/vehicle partitioning. Arch Dermatol Res. 1990;282(8):498–505.
Fartasch M. Ultrastructure of the epidermal barrier after irritation. Microsc Res Tech. 1997;37(3):193–9.
Patil S, Singh P, Sarasour K, Maibach H. Quantification of sodium lauryl sulfate penetration into the skin and underlying tissues after topical application—pharmacological and toxicological implications. J Pharm Sci. 1995;84(10):1240–4.
di Nardo A, Sugino K, Wertz P, Ademola J, Maibach HI. Sodium lauryl sulfate (SLS) induced irritant contact dermatitis: a correlation study between ceramides and in vivo parameters of irritation. Contact Dermatitis. 1996;35(2):86–91.
Lévêque JL, de Rigal J, Saint-Léger D, Billy D. How does sodium lauryl sulfate alter the skin barrier function in man? A multiparametric approach. Skin Pharmacol Physiol. 1993;6(2):111–5.
Froebe CL, Simion FA, Rhein LD, Cagan RH, Kligman A. Stratum corneum lipid removal by surfactants: relation to in vivo irritation. Dermatology. 1990;181(4):277–83.
Čuříková-Kindlová BA, Diat O, Štěpánek F, Vávrová K, Zbytovská J. Probing the interactions among sphingosine and phytosphingosine ceramides with non- and alpha-hydroxylated acyl chains in skin lipid model membranes. Int J Pharm. 2019;563:384–94.
Lodén M, Bárány E. Skin-identical lipids versus petrolatum in the treatment of tape-stripped and detergent-perturbed human skin. Acta Derm-Venereol. 2000;80(6):412–5.
Hatziantoniou S, Rallis M, Demetzos C, Papaioannou GT. Pharmacological activity of natural lipids on a skin barrier disruption model. Pharmacol Res. 2000;42(1):55–9.
De Paepe K, Roseeuw D, Rogiers V. Repair of acetone- and sodium lauryl sulphate-damaged human skin barrier function using topically applied emulsions containing barrier lipids. J Eur Acad Dermatol Venereol. 2002;16(6):587–94.
Man M-Q, Feingold KR, Elias PM. Exogenous lipids influence permeability barrier recovery in acetone-treated murine skin. Arch Dermatol. 1993;129(6):728–38.
Verma DD, Verma S, Blume G, Fahr A. Particle size of liposomes influences dermal delivery of substances into skin. Int J Pharm. 2003;258(1):141–51.
de Pera M, Coderch L, Fonollosa J, de la Maza A, Parra JL. Effect of internal wool lipid liposomes on skin repair. Skin Pharmacol Physiol. 2000;13(3–4):188–95.
Kucharekova M, Schalkwijk J, Van De Kerkhof PCM, Van De Valk PGM. Effect of a lipid-rich emollient containing ceramide 3 in experimentally induced skin barrier dysfunction. Contact Dermatitis. 2002;46(6):331–8.
Lynde CW, Andriessen A. A cohort study on a ceramide-containing cleanser and moisturizer used for atopic dermatitis. Cutis. 2014;93(4):207–13.
Sahle FF, Gebre-Mariam T, Dobner B, Wohlrab J, Neubert RHH. Skin diseases associated with the depletion of stratum corneum lipids and stratum corneum lipid substitution therapy. Skin Pharmacol Physiol. 2015;28(1):42–55.
Zhang Q, Flach CR, Mendelsohn R, Mao G, Pappas A, Mack MC, et al. Topically applied ceramide accumulates in skin glyphs. Clin Cosmet Investig Dermatol. 2015;8:329–37.
Sjövall P, Skedung L, Gregoire S, Biganska O, Clément F, Luengo GS. Imaging the distribution of skin lipids and topically applied compounds in human skin using mass spectrometry. Sci Rep. 2018;8(1):16683.
Imokawa G, Akasaki S, Minematsu Y, Kawai M. Importance of intercellular lipids in water-retention properties of the stratum corneum: induction and recovery study of surfactant dry skin. Arch Dermatol Res. 1989;281(1):45–51.
Schoenwald RD, Stewart P. Effect of particle size on ophthalmic bioavailability of dexamethasone suspensions in rabbits. J Pharm Sci. 1980;69(4):391–4.
Lippacher A, Müller RH, Mäder K. Liquid and semisolid SLNTM dispersions for topical application: rheological characterization. Eur J Pharm Biopharm. 2004;58(3):561–7.
du Plessis J, Ramachandran C, Weiner N, Müller DG. The influence of particle size of liposomes on the deposition of drug into skin. Int J Pharm. 1994;103(3):277–82.
Sakuyama S, Hirabayashi C, Hasegawa J-I, Yoshida S. Analysis of human face skin surface molecules in situ by Fourier-transform infrared spectroscopy. Skin Res Technol. 2010;16(2):151–60.
Brancaleon L, Bamberg MP, Sakamaki T, Kollias N. Attenuated total reflection–Fourier transform infrared spectroscopy as a possible method to investigate biophysical parameters of stratum corneum in vivo. J Invest Dermatol. 2001;116(3):380–6.
Boncheva M, Damien F, Normand V. Molecular organization of the lipid matrix in intact Stratum corneum using ATR-FTIR spectroscopy. Biochim Biophys Acta (BBA) - Biomembr. 2008;1778(5):1344–55.
Bernard G, Auger M, Soucy J, Pouliot R. Physical characterization of the stratum corneum of an in vitro psoriatic skin model by ATR-FTIR and Raman spectroscopies. Biochim Biophys Acta (BBA) - Gen Subj. 2007;1770(9):1317–23.
He W, Guo X, Xiao L, Feng M. Study on the mechanisms of chitosan and its derivatives used as transdermal penetration enhancers. Int J Pharm. 2009;382(1):234–43.
Schwarz JC, Klang V, Hoppel M, Mahrhauser D, Valenta C. Natural microemulsions: formulation design and skin interaction. Eur J Pharm Biopharm. 2012;81(3):557–62.
Pilgram GSK, Vissers DCJ, van der Meulen H, Koerten HK, Pavel S, Lavrijsen SPM, et al. Aberrant lipid organization in stratum corneum of patients with atopic dermatitis and lamellar ichthyosis. J Invest Dermatol. 2001;117(3):710–7.
Gorcea M, Hadgraft J, Moore DJ, Lane ME. In vivo barrier challenge and initial recovery in human facial skin. Skin Res Technol. 2013;19(1):e375–82.
Ramsing DW, Agner T. Effect of glove occlusion on human skin (II). Contact Dermatitis. 1996;34(4):258–62.
Zhai H, Maibach HI. Occlusion vs. skin barrier function. Skin Res Technol. 2002;8(1):1–6.
Iikura H, Uchida K, Ogawa-Fuse C, Bito K, Naitou S, Hosokawa M, et al. Effects of temperature and humidity on the skin permeation of hydrophilic and hydrophobic drugs. AAPS PharmSciTech. 2019;20(7):264.
Acknowledgements
This study was financially supported by the Czech Science Foundation (GACR19-09600S). Evonik is gratefully acknowledged for donating ceramides. We would like to thank Radek Stibor for providing the porcine skin.
Funding
This study was financially supported by the Czech Science Foundation (project GACR19-09600S).
Author information
Authors and Affiliations
Contributions
BAC-K performed the permeation and FTIR experiments, analyzed the data, and wrote the manuscript. AV prepared the samples, performed the permeation experiments, and reviewed the manuscript. AN performed the DSC measurements, evaluated the data, and reviewed the manuscript. JZ conceived the project, designed the experiments, analyzed the data, and wrote the manuscript. The authors have read the manuscript and approved its submission.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Čuříková-Kindlová, B.A., Vovesná, A., Nováčková, A. et al. In Vitro Modeling of Skin Barrier Disruption and its Recovery by Ceramide-Based Formulations. AAPS PharmSciTech 23, 21 (2022). https://doi.org/10.1208/s12249-021-02154-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-02154-z