Abstract
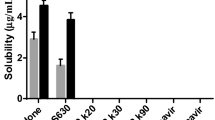
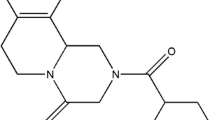
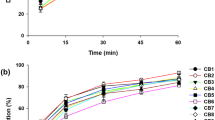
Being a candidate of BCS class II, dolutegravir (DTG), a recently approved antiretroviral drug, possesses solubility issues. The current research was aimed to improve the solubility of the DTG and thereby enhance its efficacy using the solid dispersion technique. In due course, the miscibility study of the drug was performed with different polymers, where Poloxamer 407 (P407) was found suitable to move forward. The solid dispersion of DTG and P407 was formulated using solvent evaporation technique with a 1:1 proportion of drug and polymer, where the solid-state characterization was performed using differential scanning calorimetry, Fourier transform infrared spectroscopy and X-ray diffraction. No physicochemical interaction was found between the DTG and P407 in the fabricated solid dispersion; however, crystalline state of the drug was changed to amorphous as evident from the X-ray diffractogram. A rapid release of DTG was observed from the solid dispersion (>95%), which is highly significant (p<0.05) as compared to pure drug (11.40%), physical mixture (20.07%) and marketed preparation of DTG (35.30%). The drug release from the formulated solid dispersion followed Weibull model kinetics. Finally, the rapid drug release from the solid dispersion formulation revealed increased Cmax (14.56 μg/mL) when compared to the physical mixture (4.12 μg/mL) and pure drug (3.45 μg/mL). This was further reflected by improved bioavailability of DTG (AUC: 105.99±10.07 μg/h/mL) in the experimental Wistar rats when compared to the AUC of animals administered with physical mixture (54.45±6.58 μg/h/mL) and pure drug (49.27±6.16 μg/h/mL). Therefore, it could be concluded that the dissolution profile and simultaneously the bioavailability of DTG could be enhanced by means of the solid dispersion platform using the hydrophilic polymer, P407, which could be projected towards improved efficacy of the drug in HIV/AIDS.








Similar content being viewed by others
References
Wong K, Nguyen J, Blair L, Banjanin M, Grewal B, Bowman S, et al. Pathogenesis of human immunodeficiency virus-mycobacterium tuberculosis co-infection. J Clin Med. 2020;9(11). https://doi.org/10.3390/jcm9113575.
WHO Guidelines Approved by the Guidelines Review Committee. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. Geneva: World Health Organization Copyright © World Health Organization 2017.; 2017.
Akimbekov NS, Ortoski RA, Razzaque MS. Effects of sunlight exposure and vitamin D supplementation on HIV patients. J Steroid Biochem Mol Biol. 2020;200:105664. https://doi.org/10.1016/j.jsbmb.2020.105664.
Heron JE, Norman SM, Yoo J, Lembke K, O’Connor CC, Weston CE, et al. The prevalence and risk of non-infectious comorbidities in HIV-infected and non-HIV infected men attending general practice in Australia. PLoS One. 2019;14(10):e0223224. https://doi.org/10.1371/journal.pone.0223224.
Carpenter CC, Cooper DA, Fischl MA, Gatell JM, Gazzard BG, Hammer SM, et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. JAMA. 2000;283(3):381–90. https://doi.org/10.1001/jama.283.3.381.
Endsley AN, Ho RJ. Enhanced anti-HIV efficacy of indinavir after inclusion in CD4-targeted lipid nanoparticles. J Acquir Immune Defic Syndr. 2012;61(4):417–24. https://doi.org/10.1097/QAI.0b013e3182653c1f.
Kakuda TN, Page LM, Anderson PL, Henry K, Schacker TW, Rhame FS, et al. Pharmacological basis for concentration-controlled therapy with zidovudine, lamivudine, and indinavir. Antimicrob Agents Chemother. 2001;45(1):236–42. https://doi.org/10.1128/aac.45.1.236-242.2001.
Ene L, Duiculescu D, Ruta SM. How much do antiretroviral drugs penetrate into the central nervous system? J Med Life. 2011;4(4):432–9.
Robillard KR, Chan GN, Zhang G, la Porte C, Cameron W, Bendayan R. Role of P-glycoprotein in the distribution of the HIV protease inhibitor atazanavir in the brain and male genital tract. Antimicrob Agents Chemother. 2014;58(3):1713–22. https://doi.org/10.1128/aac.02031-13.
Zhao XZ, Smith SJ, Maskell DP, Metifiot M, Pye VE, Fesen K, et al. HIV-1 integrase strand transfer inhibitors with reduced susceptibility to drug resistant mutant integrases. ACS Chem Biol. 2016;11(4):1074–81. https://doi.org/10.1021/acschembio.5b00948.
Lakshman D, Chegireddy M, Hanegave GK, Sree KN, Kumar N, Lewis SA, et al. Investigation of drug-polymer miscibility, biorelevant dissolution, and bioavailability improvement of Dolutegravir-polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer solid dispersions. Eur J Pharm Sci. 2020;142:105137. https://doi.org/10.1016/j.ejps.2019.105137.
Pandey M, Choudhury H, Verma RK, Chawla V, Bhattamisra SK, Gorain B, et al. Nanoparticles based intranasal delivery of drug to treat Alzheimer’s disease: a recent update. CNS Neurol Disord Drug Targets. 2020;19:648–62. https://doi.org/10.2174/1871527319999200819095620.
Choudhury H, Pandey M, Chin PX, Phang YL, Cheah JY, Ooi SC, et al. Transferrin receptors-targeting nanocarriers for efficient targeted delivery and transcytosis of drugs into the brain tumors: a review of recent advancements and emerging trends. Drug Deliv Transl Res. 2018;8(5):1545–63. https://doi.org/10.1007/s13346-018-0552-2.
Jacob S, Nair AB. Cyclodextrin complexes: perspective from drug delivery and formulation. Drug Dev Res. 2018;79(5):201–17. https://doi.org/10.1002/ddr.21452.
Kamboj S, Bala S, Nair AB. Solid lipid nanoparticles: an effective lipid based technology for poorly water soluble drugs. Int J Pharm Sci Rev Res. 2010;5(2):78–90.
Paudwal G, Rawat N, Gupta R, Baldi A, Singh G, Gupta PN. Recent advances in solid dispersion technology for efficient delivery of poorly water-soluble drugs. Curr Pharm Des. 2019;25(13):1524–35. https://doi.org/10.2174/1381612825666190618121553.
Huang Y, Dai WG. Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm Sin B. 2014;4(1):18–25. https://doi.org/10.1016/j.apsb.2013.11.001.
Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006;23(12):2709–28. https://doi.org/10.1007/s11095-006-9104-4.
Charbe N, Baldelli S, Cozzi V, Castoldi S, Cattaneo D, Clementi E. Development of an HPLC-UV assay method for the simultaneous quantification of nine antiretroviral agents in the plasma of HIV-infected patients. J Pharm Anal. 2016;6(6):396–403. https://doi.org/10.1016/j.jpha.2016.05.008.
Nair AB, Kumria R, Al-Dhubiab BE, Attimarad M, Harsha S. Development of transdermal delivery system of vildagliptin and its comparison with oral therapy. Indian J Pharm Educ Res. 2016;50(1):130–7. https://doi.org/10.5530/ijper.50.1.17.
Nair AB, Attimarad M, Al-Dhubiab BE, Wadhwa J, Harsha S, Ahmed M. Enhanced oral bioavailability of acyclovir by inclusion complex using hydroxypropyl-β-cyclodextrin. Drug Delivery. 2014;21(7):540–7. https://doi.org/10.3109/10717544.2013.853213.
Higuchi T, Lach JL. Investigation of some complexes formed in solution by caffeine. IV. Interactions between caffeine and sulfathiazole, sulfadiazine, p-aminobenzoic acid, benzocaine, phenobarbital, and barbital. J Am Pharm Assoc Am Pharm Assoc. 1954;43(6 1):349–54. https://doi.org/10.1002/jps.3030430609.
Shah J, Vasanti S, Anroop B, Vyas H. Enhancement of dissolution rate of valdecoxib by solid dispersions technique with PVP K 30 & PEG 4000: preparation and in vitro evaluation. J Incl Phenom Macrocycl Chem. 2009;63(1-2):69–75. https://doi.org/10.1007/s10847-008-9490-9.
Jacob S, Shirwaikar A, Nair A. Preparation and evaluation of fast-disintegrating effervescent tablets of glibenclamide. Drug Dev Ind Pharm. 2009;35(3):321–8. https://doi.org/10.1080/03639040802337021.
Harsha SN, Aldhubiab BE, Nair AB, Alhaider IA, Attimarad M, Venugopala KN, et al. Nanoparticle formulation by Büchi b-90 nano spray dryer for oral mucoadhesion. Drug Des Devel Ther. 2015;9:273–82. https://doi.org/10.2147/DDDT.S66654.
SreeHarsha N, Hiremath JG, Sarudkar S, Attimarad M, Al-Dhubiab B, Nair AB, et al. Spray dried amorphous form of simvastatin: preparation and evaluation of the buccal tablet. Indian J Pharm Educ Res. 2020;54(1):46–54.
Choudhury H, Gorain B, Karmakar S, Biswas E, Dey G, Barik R, et al. Improvement of cellular uptake, in vitro antitumor activity and sustained release profile with increased bioavailability from a nanoemulsion platform. Int J Pharm. 2014;460(1-2):131–43. https://doi.org/10.1016/j.ijpharm.2013.10.055.
Shah H, Nair AB, Shah J, Bharadia P, Al-Dhubiab BE. Proniosomal gel for transdermal delivery of lornoxicam: optimization using factorial design and in vivo evaluation in rats. Daru : journal of Faculty of Pharmacy, Tehran University of Medical Sciences. 2019;27(1):59–70. https://doi.org/10.1007/s40199-019-00242-x.
Nagaraja SH, Al-Dhubiab BE, Tekade RK, Venugopala KN, Ghorpade RV, Meravanige G, et al. Novel preparation and effective delivery of mucoadeshive nanoparticles containing anti-diabetic drug. Indian J Pharm Educ Res. 2019;53(2):S43–S9. https://doi.org/10.5530/ijper.53.2s.47.
Nair AB, Al-Dhubiab BE, Shah J, Jacob S, Saraiya V, Attimarad M, et al. Mucoadhesive buccal film of almotriptan improved therapeutic delivery in rabbit model. Saudi Pharm J. 2020;28(2):201–9. https://doi.org/10.1016/j.jsps.2019.11.022.
Anderson NH, Bauer M, Boussac N, Khan-Malek R, Munden P, Sardaro M. An evaluation of fit factors and dissolution efficiency for the comparison of in vitro dissolution profiles. J Pharm Biomed Anal. 1998;17(4-5):811–22. https://doi.org/10.1016/s0731-7085(98)00011-9.
Jhaveri M, Nair AB, Shah J, Jacob S, Patel V, Mehta T. Improvement of oral bioavailability of carvedilol by liquisolid compact: optimization and pharmacokinetic study. Drug Deliv Transl Res. 2020;10(4):975–85.
Akrawi SH, Gorain B, Nair AB, Choudhury H, Pandey M, Shah JN, et al. Development and optimization of naringenin-loaded chitosan-coated nanoemulsion for topical therapy in wound healing. Pharmaceutics. 2020;12(9). https://doi.org/10.3390/pharmaceutics12090893.
Nair A, Morsy MA, Jacob S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev Res. 2018;79(8):373–82. https://doi.org/10.1002/ddr.21461.
Tian Y, Booth J, Meehan E, Jones DS, Li S, Andrews GP. Construction of drug-polymer thermodynamic phase diagrams using Flory-Huggins interaction theory: identifying the relevance of temperature and drug weight fraction to phase separation within solid dispersions. Mol Pharm. 2013;10(1):236–48. https://doi.org/10.1021/mp300386v.
Marsac PJ, Li T, Taylor LS. Estimation of drug-polymer miscibility and solubility in amorphous solid dispersions using experimentally determined interaction parameters. Pharm Res. 2009;26(1):139–51. https://doi.org/10.1007/s11095-008-9721-1.
Sankari T, Al-Hariri S. Preparation and characterization of cefuroxime axetil solid dispersions using poloxamer 188. Braz J Pharm Sci. 2018;54(4).
Bodratti AM, Alexandridis P. Formulation of Poloxamers for Drug Delivery. J Funct Biomater. 2018;9(1). https://doi.org/10.3390/jfb9010011.
Chaudhary AS, Chaudhary BA, Mehta AT. Formulation development and optimization of polyox based quick dissolving film of quetiapine. J Pharm Bioallied Sci. 2012;4(Suppl 1):S19–20. https://doi.org/10.4103/0975-7406.94123.
Janssens S, Van den Mooter G. Review: physical chemistry of solid dispersions. J Pharm Pharmacol. 2009;61(12):1571–86. https://doi.org/10.1211/jpp/61.12.0001.
Tran TTD, Tran PHL. Molecular interactions in solid dispersions of poorly water-soluble drugs. Pharmaceutics. 2020;12(8). https://doi.org/10.3390/pharmaceutics120807458.
Gonjo T, Futami Y, Morisawa Y, Wojcik MJ, Ozaki Y. Hydrogen bonding effects on the wavenumbers and absorption intensities of the OH fundamental and the first, second, and third overtones of phenol and 2,6-dihalogenated phenols studied by visible/near-infrared/infrared spectroscopy. J Phys Chem A. 2011;115(35):9845–53. https://doi.org/10.1021/jp201733n.
Takano R, Furumoto K, Shiraki K, Takata N, Hayashi Y, Aso Y, et al. Rate-limiting steps of oral absorption for poorly water-soluble drugs in dogs: prediction from a miniscale dissolution test and a physiologically-based computer simulation. Pharm Res. 2008;25(10):2334–44. https://doi.org/10.1007/s11095-008-9637-9.
Singh G, Sharma S, Gupta GD. Extensive diminution of particle size and amorphization of a crystalline drug attained by eminent technology of solid dispersion: a comparative study. AAPS PharmSciTech. 2017;18(5):1770–84. https://doi.org/10.1208/s12249-016-0647-3.
Jacob S, Nair AB. An updated overview with simple and practical approach for developing in vitro-in vivo correlation. Drug Dev Res. 2018;79(3):97–110. https://doi.org/10.1002/ddr.21427.
Nair AB, Al-Dhubiab BE, Shah J, Attimarad M, Harsha S. Poly(Lactic acid-co-glycolic acid) nanospheres improved the oral delivery of Candesartan Cilexetil. Indian J Pharm Educ Res. 2017;51(4):571–9. https://doi.org/10.5530/ijper.51.4.86.
Acknowledgments
The authors are highly grateful to Emcure Pharmaceuticals Ltd., Ahmedabad, India, for donating gift samples of drugs and polymers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chaudhary, S., Nair, A.B., Shah, J. et al. Enhanced Solubility and Bioavailability of Dolutegravir by Solid Dispersion Method: In Vitro and In Vivo Evaluation—a Potential Approach for HIV Therapy. AAPS PharmSciTech 22, 127 (2021). https://doi.org/10.1208/s12249-021-01995-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-021-01995-y